
University of California, Irvine
Instructor: Dr. Barbara J. Becker

|
|
|
|
It is hard now to realize the state of puzzled mystery in which we lived in those days... |
When a scientific paradigm is in flux--
What are legitimate scientific research questions? |
|
|
In astronomy, human ingenuity will, probably, in future, be able to accomplish little more than an improvement in the means of making observations, or in the analysis by which the rules of computation are investigated. We can imagine the possibility of determining the shapes of stars, their distances, their sizes, and their movements; whereas there is no means by which we will ever be able to examine their chemical composition, their mineralogical structure, or especially, the nature of organisms that live on their surfaces.... Our positive knowledge with respect to the stars is necessarily limited to their observed geometrical and mechanical behavior. |
|
|
 |
|
|
|
|
|

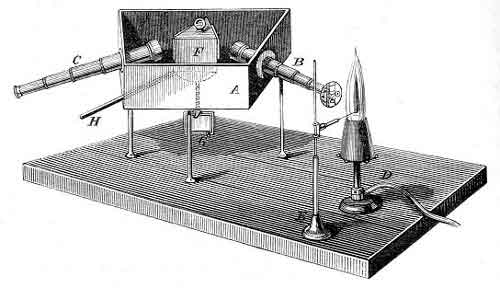
William Huggins (1824-1910) |
|
|
 |
|
|
|
Johannes Kepler (1571-1630) was convinced there was a mathematical plan underlying the structure of the system of planets orbiting the sun. He was particularly vexed by the challenge of explaining the enormous distance separating the planets Mars and Jupiter. Why is it this way and not otherwise?? In 1702, David Gregory (1659-1708) described the relative distances of the known planets from the sun as proportional to the numbers: In 1724, philosopher Christian von Wolff (1679-1754) reproduced Gregory's series in a publication that was later read by physicist, Johann Daniel Titius (1729-1796). In 1766, Titius modified the series to create a neat mathematical progression: 4, 4 + 3 = 7, 4 + 6 = 10, 4 + 12 = 16, 4 + 48 = 52, and 4 + 96 = 100. Expressing the series this way drew renewed attention to the gap between the 4th and 5th terms, a gap that the numbers seemed to decree ought to be filled by something orbiting the sun at a distance proportional to 4 + 24 = 28. Titius's series reached a much wider audience after it appeared in the 1772 edition of a popular introductory astronomy text by Johann Elert Bode (1747-1826). Nevertheless, it remained little more than an intellectual curiosity until 1781, when William Herschel (1738-1822) announced his serendipitous discovery of a new planet (Uranus) orbiting the sun at a distance roughly proportional to the next number in the Bode-Titius sequence: 4 + 192 = 196! In 1800, a group of determined astronomers launched a team effort to conduct a systematic search for the missing planet. Meanwhile, a Sicilian astronomer, Giuseppe Piazzi (1746-1826), was pursuing his own research project: using a newly acquired precision sighting instrument to create a new and more accurate star catalogue. On January 1, 1801, he noted the coordinates of a dim and previously unrecorded star. The next night, during a routine check to confirm his previous night's measures, Piazzi found, to his surprise, that this little star had moved! Subsequent observations assured him that the object was not a fixed star, but a satellite of the sun, perhaps a comet. Piazzi worked at determining the body's orbital path. When comets are near the sun, their orbits resemble parabolas. But Piazzi found the object did not follow a parabolic path. Instead, its coordinates seemed to fit a near circular orbit with a radius 2.7 times that of Earth's. After Piazzi's discovery was affirmed by other astronomers, he named the new planet Ceres after the Roman goddess of agriculture who was the patron goddess of Sicily. Attempts to observe and measure Ceres' diameter proved difficult and it soon became clear that this was no ordinary planet, but rather an extremely small body, probably much smaller than the Moon. Then, in March 1802, another new "planet" was discovered by Heinrich Olbers (1758-1840). Named Pallas (after Athena, the Greek goddess of wisdom), this second body was estimated to be even smaller than Ceres, although the two appeared to share roughly the same orbit. William Herschel suggested calling Ceres and Pallas "asteroids" to distinguish them from the rest of the planets. How many of this new breed of solar satellite were out there? Had a larger planet been broken into chunks in some ancient calamitous event? Astronomers searched for signs of more of these tiny celestial bodies. Juno (sister and wife of the Roman god Jupiter) was found in 1804 by Karl Ludwig Harding (1765-1834), and Olbers spotted his second asteroid, Vesta (Roman goddess of the hearth), in 1807. The asteroids' small size made them challenging objects to find, and the next one, Astraea (Greek goddess of justice), was not discovered until 1845, by Karl Ludwig Hencke (1793-1866). In that year, another planetary puzzle emerged. This one involved the planet Uranus. Although it had only recently been recognized as a planet, Uranus had actually been recorded as a star on maps as early as 1690. Astronomers used this information to track the planet's motion against the background of fixed stars over this lengthy period. They found its actual path deviated significantly from the trajectory prescribed for it by Newton's law of universal gravitation. Were there inaccuracies in the early star maps? Is Newton's law flawed in some way? Or is there, perhaps, another previously unobserved body orbiting the sun at a distance proportional to the next number in the Bode-Titius series (4 + 384 = 388) which could account for the perturbations in Uranus's orbital path? Assuming this value for the hypothetical distance of the perturbing planet, two astronomers--Urbain Jean Joseph Le Verrier (1811-1877) at the Paris Observatory, and John Couch Adams (1819-1892) at Cambridge University--independently calculated its likely location. Guided by Le Verrier's figures, Johann Gottfried Galle (1812-1910) at the Berlin Observatory quickly found a likely suspect on September 23, 1846. When astronomers determined the orbit of this new planet, Neptune, they found it did not match the value assigned it by the Bode-Titius series. What role, then, can we say that the Bode-Titius series actually played in the discovery of Neptune? |
The Bode-Titius Series |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
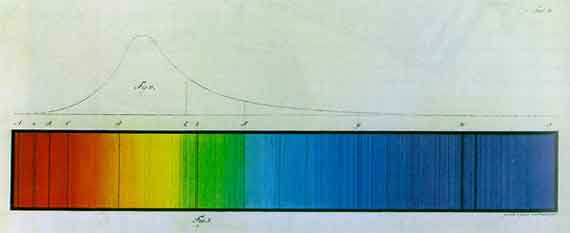
| Johann Jacob Balmer (1825-1898)
"Note on the Spectral Lines of Hydrogen," Annalen der Physik (1885) Ångström's very exact measurements of the four hydrogen lines enable one to determine a common factor [3645.6 ¥ 10-10 m] for their wavelengths which is in as simple a numerical relation as possible to these wavelengths.... Red (Ha)
-- 6562.10 ¥ 10-10
m
I gradually arrived at a formula which, at least for these four lines, expresses a law by which their wavelengths can be represented with striking precision.... |

|
Balmer's series of "simple numerical relations" -- 9 , 4 , 25 , 9 ....Multiply the 2nd and 4th members of this series by 4/4 -- 9 , 16 , 25 , 36 ....to generate a new series that can now be extended-- 9 , 16 , 25 , 36 , 49 , 64 , 81 ....based on the following generalized pattern-- where n = 3, 4, 5 ....Multiplying the numbers in Balmer's series by 3645.6 ¥ 10-10 mgenerates the wavelengths of hydrogen's spectral lines. What physical meaning, if any, lies behind this curious series? |
|
|
|
| Wilhelm
Roentgen (1845-1923)
November 1895. When electricity flows through a vacuum tube, penetrating invisible radiation is emitted. |
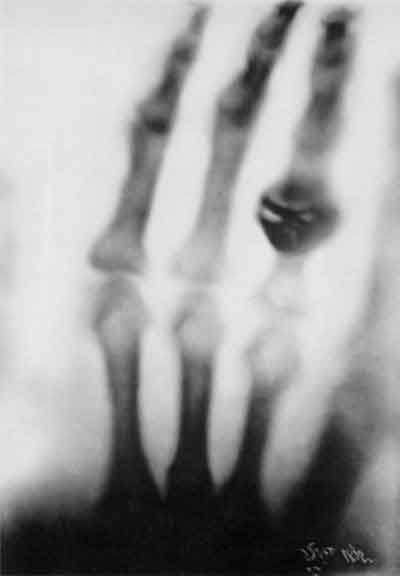
Shadowgraph of Frau Roentgen's hand created by "x-rays"-- December 22, 1895. |
 |
Henri Becquerel (1852-1908)
February 1896. Uranium salts spontaneously emit invisible rays that are similar to x-rays, although not as penetrating. |
| Marie Sklodovska Curie (1867-1934)
February 1898. Uranium is not the only natural emitter of penetrating radiation. "Radioactivity," like magnetism, is a property possessed by a select group of elements. |
 |
 |
December 1898. Pitchblende,
a uranium ore, is more radioactive than pure uranium!!
Curie isolated a new element in the pitchblende, polonium, and another, even more potent source of radioactivity: radium. |
 |
Joseph John Thomson (1856-1940)
1899. |
| Marie at work with
Pierre Curie (1859-1906) 1902.
1903.
|
 |
The New York Times
- January 14, 1904 - TO MAKE LUMINOUS
______________ Drs. Kunz and Merton Discuss
______ Interiors of Patients May be Lighted
of Disease __________ Radium and actinium were discussed last night before the Technology Club of New York in the operating rooms of Dr. William J. Morton, 10 East Twenty-eighth Street, by Dr. George F. Kunz and Dr. Morton, who is Professor of Electro-Therapeutics in the New York Post-Graduate Medical School and Hospital. Dr. Kunz reviewed the experiments which have been made with radium in Paris from a metallurgical point of view. He told of the many interesting qualities of pitchblende, taken from Pribam, in Austria, which he asserted, resembled radium in many respects. The doctor had with him a specimen of radium, and he also exhibited a sample of American radium ore which was discovered in Colorado. His exhibits also included a sample of actinium, which he said was the first specimen of that ore ever displayed in public. In order to carry out the experiments it was necessary to extinguish all the lights in the room. That being done Dr. Kunz took from his pocket a small piece of radium about the size of a pin head, which he said was of 100,000 activity, and had cost nearly $300. The radium was inclosed in a glass tube, outside of which there were four other tubes of copper, iron, rubber, and lead. He then produced the famous "Tiffany diamond," weighing more than 15 carats. In order to show the luminous properties of the radium he held the diamond about two inches away from the tubes, and exhibited it to the audience. The light seen was like that of phosphorus. It would have made no difference, the doctor said, if the tubes had been a foot thick, the glow would have been discernible. Afterward the diamond was placed in a glass of water, where it shone beautifully. The bombardment of radium was demonstrated by means of the spintharescope, and it looked like a tiny Roman candle throwing off a myriad of sparks. Dr. Kunz said he thought that in time radium of a very high activity would be obtainable, and he would not be surprised if it reached 1,500,000. Dr. Morton explained in detail the uses to which radium might be put in curing diseases, particularly those of an internal nature. "Medicine," he said, "is gradually abandoning its old-fashioned concoctions, and we are taking up radium with exceedingly bright prospects. Its use will consist of physical treatment almost exclusively. The Roentgen ray has been of immense value in curing cancer, but radium promises to go far ahead of it. If we had radium of 150,000 activity we could no doubt do a great deal more than we are doing now. Most of us have been confined to a much lower radio-activity. We have been working with from 7,000 to 10,000 luminosity. "The actual glow of radium does not represent its actual radio activity. There is a great difference in the ore. One sort of radium may possess a high luminosity, while another sort may have a high radio-activity and very little luminosity. We cannot boast of the luminosity of the kind which we now have." Dr. Morton startled his hearers by telling of a mixture which he had prepared and called "liquid sunshine," the name having been applied because the Doctor regarded it a good "catch" phrase to give to the preparation. By means of this fluid, he said, the whole interior of a patient could be lighted up. The doctor exhibited six tubes containing "liquid sunshine," one of which, he explained, contained quinine sulphate which had been exposed to radio activity. He then proceeded to show the luminous quality of the fluid by placing each tube before a strong X ray, whereupon a spot of faint light was seen abut the size of the palm of the human hand. "That," said the doctor, "would be the result if the liquid were taken inside. I believe," he added. "that radium may after all be the real curative property which has been found in so many spring waters throughout the world. "The advantage of radium over the X ray is that it can be applied direct to the part affected. For example, if placed in a small tube it may be inserted in the throat, and in similar manner it may be applied to any vital region. In other works, with radium we shall be able to get at the seat of diseases. There is no end, in my opinion, to the cures which may be effected by radio-activity, excited in one way or another. "In imparting radio-activity to liquids, however, we will have to be extremely careful, and physicians will need to use the utmost discretion in advising patients to drink the fluid. It will be possible, however, to bathe a patient's entire interior in violet or ultra violet light as the result of this discovery, and this light we have decided to call 'sunshine.' We know of the value of sunshine on the outside, particularly where bald heads are concerned, and we believe it will have a similar effect on the inside." Dr. Morton told of several cures of cancer by radium, and exhibited a bell-shaped glass, where the smaller tubes of radium, of about 7,000 activity, could be placed in the flesh affected. As the activities of radium became greater, he expected that more important results would follow.
|
|
|
|
| Max Planck (1857-1947)
1900
"My futile attempts to fit the ... quantum ... somehow into the classical theory continued for a number of years, and they cost me a great deal of effort." |
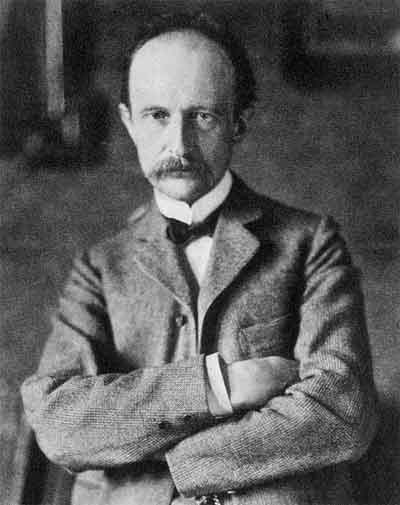 |
 |
Hans Geiger (1882-1945) and Ernest Rutherford (1871-1937)
1911
|
1938
Not only is energy released in this remarkable process, but, as Irène Curie--daughter of Marie and Pierre Curie--and her husband Frédéric soon showed, nuclear fission produces additional neutrons that can go on to bombard other uranium atoms that may be nearby. Imagine a room filled with mousetraps, each set with a ping pong ball resting on its surface. What will happen when you toss another ping pong ball into the room? |
|
|
The World Set Free (1914) by H. G. Wells Prelude, Section 8 'And so,' said the professor, 'we see that this Radium ... is an element that is breaking up and flying to pieces. But perhaps all elements are doing that at less perceptible rates. Uranium certainly is; thorium--the stuff of this incandescent gas mantle -- certainly is; actinium. I feel that we are but beginning the list. And we know now that the atom, that once we thought hard and impenetrable, and indivisible and final and -- lifeless -- lifeless, is really a reservoir of immense energy. That is the most wonderful thing about all this work. A little while ago we thought of the atoms as we thought of bricks, as solid building material, as substantial matter, as unit masses of lifeless stuff, and behold! these bricks are boxes, treasure boxes, boxes full of the intensest force. This little bottle contains about a pint of uranium oxide; that is to say, about fourteen ounces of the element uranium. It is worth about a pound. And in this bottle, ladies and gentlemen, in the atoms in this bottle there slumbers at least as much energy as we could get by burning a hundred and sixty tons of coal. If at a word, in one instant I could suddenly release that energy here and now it would blow us and everything about us to fragments; if I could turn it into the machinery that lights this city, it could keep Edinburgh brightly lit for a week. But at present no man knows, no man has an inkling of how this little lump of stuff can be made to hasten the release of its store. It does release it, as a burn trickles. Slowly the uranium changes into radium, the radium changes into a gas called the radium emanation, and that again to what we call radium A, and so the process goes on, giving out energy at every stage, until at last we reach the last stage of all, which is, so far as we can tell at present, lead. But we cannot hasten it.' |
| 1934 | Irène Joliot-Curie (1897-1956) and Frédéric Joliot (1900-1958) found that it is possible to induce radioactivity in normally stable elements. Bombarding aluminum (atomic number = 13) with alpha particles (helium nuclei, comprised of 2 protons and 2 neutrons) caused some of the aluminum nuclei to capture an alpha particle. Now unstable, these nuclei immediately emitted a neutron, thus producing a new element--a radioactive isotope of phosphorus (at. no. = 15). One no longer had to rely solely on natural materials for sources of radioactivity--radioactive elements could be manufactured in the laboratory. Perhaps their rate of radioactive emission could be controlled! |
| 1938 | Otto Hahn (1879-1968) and Fritz Strassmann (1902-1980) announced the surprising observation that bombarding uranium (at. no. = 92) with neutrons did not produce radium atoms (at. no. = 88) as expected. Instead, the product was an element with a considerably lower atomic number--barium (at. no. = 56). Hahn and Strassman suspected that the uranium nuclei were being broken into two nearly equal parts, a process they called "fission". The small amount of mass that is lost every time this occurs is converted to approximately 200 million electron volts of energy. |
| 1939 | Niels Bohr (1885-1962) announced results of an experiment by Lise Meitner (1878-1968) and Otto Frisch (1904-1979) which confirmed Hahn and Strassman's discovery. |

Lise Meitner (1878-1968) and Otto Hahn (1879-1968) |
|
|
2 August 1939 Some recent work by E. Fermi and L. Szilard, which has been communicated to me in manuscript, leads me to expect that the element uranium may be turned into a new and important source of energy in the immediate future.... In the course of the last four months [research has shown] that it may become possible to set up a nuclear chain reaction in a large mass of uranium, by which vast amounts of power and large quantities of new radium-like elements would be generated. Now it appears almost certain that this could be achieved in the immediate future. This new phenomenon would also lead to the construction of bombs, and it is conceivable--though much less certain--that extremely powerful bombs of a new type may thus be constructed. A single bomb of this type ... might very well destroy [a] whole port together with some of the surrounding territory.... |
|
| 1939 | FDR formed an ad hoc committee on uranium |
| 1940 | National Defense Research Committee established to mobilize atomic research |
| 1941 | Otto Frisch and Rudolf Peierls (1907-1995) calculate that a small amount
of fissionable uranium can produce an explosion equal to several thousand
tons of TNT
National Academy of Science urges all-out effort to build atomic weapons US enters World War II |
| 1942 | research program reorganized and given over to the War Department;
code name: Manhattan Engineer District
research conference held in summer at UC Berkeley to work out theoretical details of nuclear weapon Robert Oppenheimer named director of Manhattan Engineer District Los Alamos, New Mexico selected as research site for project team working under Enrico Fermi at the University of Chicago produce first controlled chain reaction |
| 1943 | nuclear reactors begin production of sufficient fissionable material
for atomic weapon
research begins at Los Alamos on creation of "the gadget" experiments begin when first plutonium arrives at Los Alamos |
| 1945 | April 12--FDR dies of cerebral hemorrhage
May 8--VE day July 16--test of "the gadget" in the Jornado del Muerto desert in New Mexico |

The first bomb--nicknamed "The Gadget"--to be constructed on the principles of nuclear fission is detonated in Alamogordo, New Mexico on July 16, 1945. |
|
| 1945 | August 7--uranium weapon dropped on Hiroshima August 9--plutonium weapon dropped on Nagasaki August 15--VJ day |
| 1951 | physicist Max Born pondered the implications of the weapon's use:
It seems to me that the scientists who led the way to the atomic bomb were extremely skillful and ingenious, but not wise men. They delivered the fruits of their discoveries unconditionally into the hands of politicians and soldiers; thus they lost their moral innocence and their intellectual freedom.... |
|
|
|