
University of California, Irvine
Instructor: Dr. Barbara J. Becker

|
|
|
|
Subtle fluids possess physical properties that are unlike ordinary matter:
|

Onionskin Atom--
|
Subtle fluids served as fruitful model for experimental investigation:
-electric charge -heat capacity -temperature -chemical affinity.... |
|
|
| Subtle fluid model increased interest in the study of the properties and behavior of air. |
Robert Boyle (1627-1691)
|
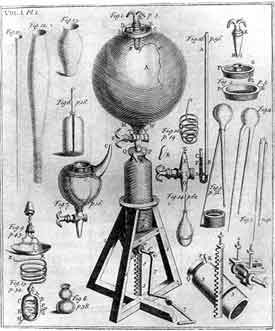
|
|
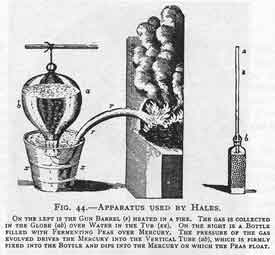
Stephen Hales (1677-1761)
|
|
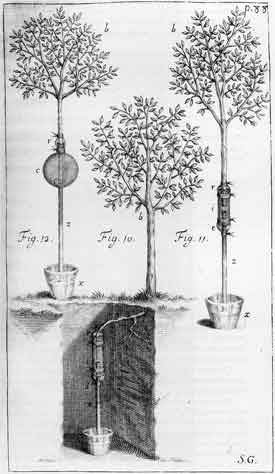 |
|
Joseph Black (1728-1799)
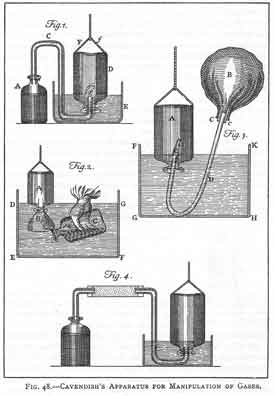
-extinguishes flame -denser than ordinary air -turns lime water milky Henry Cavendish (1731-1810)
|
Joseph Priestley (1733-1804)
needs more heat and light; supports combustion |
 |
|
|
| The principle of combustion, phlogiston (from the Greek word
phlogizein,
which means "to burn"), is fixed in the fuel.
Wood burned slowly over a long period of time by a controlled fire will absorb the stuff of fire and become charcoal, a fuel that is almost pure phlogiston. We can tell that the charcoal contains a high percentage of phlogiston because while it is being consumed, copious amounts of phlogiston are given off into the air in the form of heat and light. When all the phlogiston is exhausted, the only thing left is a small amount of ash. charcoal (stored phlogiston) + fire --> heat and light (free phlogiston) + ash When ore (an earthy substance depleted of phlogiston) is heated with charcoal (a rich source of stored phlogiston) in a closed container, it absorbs and fixes the free phlogiston released by the charcoal and returns to its metallic state. calx (ore) + charcoal (stored phlogiston) + fire --> metal (fixed phlogiston) + ash Ore weighs more than resulting metal.
Phlogiston must have negative weight! |
|
|
Antoine Lavoisier (1743-1794) It is difficult to understand heat except as the effects caused by a real and material substance--a very special elastic and subtle fluid that fills all the tiny spaces between the particles in a body and separates those particles from each other. Subtle fluid model for heat generated new knowledge and accounted for old problems: Why does the temperature of the water/ice mix remain constant during the melting process?Joseph Black (1728-1799) Heat is required to melt the ice so it is unable to raise the temperature of the water until the melting is complete. Why do bodies expand when heated?Caloric is self-repulsive. A body will expand as it becomes filled with caloric and contract when caloric is removed.
How do cold things get warm?Like water, caloric flows from an object with an excess quantity of the fluid and saturates neighboring objects that lack caloric. Heat produced by friction, hammering, or shaking is the result of caloric being squeezed or forced out of a body. |
|
|
| If caloric is a "thing", what are its measurable properties? |

Benjamin Thompson (1753-1814)
|
Count Rumford was a testy, unlikable fellow.
He was born Benjamin Thompson in 1753 on his family's farm in Woburn, Massachusetts--then a colony of Britain. Benjamin was less than two years old when his father died. His mother soon remarried and raised a large family. When he was only 13 years old, Benjamin was sent off to work in a nearby town to help support his family. A bright and curious boy, he wondered about many things during these years--the properties of wind, light, color, and heat. He wrote his many questions down in notebooks for future reference and even started a "science society" with a friend, Loammi Baldwin. |
At the age of 17, Benjamin was taken on as an apprentice by a physician in his hometown. Like most children growing up in colonial America, he had received no formal education. But his apprenticeship provided him with opportunities to conduct investigations necessary to satisfy his curiosity. When Benjamin was 20 years old, he met and married a wealthy widow. A daughter, Sarah, was born. The year was 1774, and no doubt he and his family would have lived out their lives uneventfully in Massachusetts if world events hadn't intervened. Citizens in the American colonies were growing increasingly angry with Britain's control over their everyday affairs. There was more and more talk of taking action to free the colonies from British rule. Benjamin Thompson did not agree with these rebellious ideas. He was, after all, the son-in-law of a prominent landowner with strong British ties, and he had served the Royal governor of New Hampshire as a recruiter for the British army. Even before hostilities began in 1775, Thompson listened in on his neighbors' revolutionary plans and passed the information along to British authorities, writing letters in invisible ink to avoid detection! His activities aroused more and more suspicion among those sympathetic to the rebel cause, and Benjamin fled to England in March 1776, leaving behind his wife and young daughter. At the close of the American Revolution in 1783, Benjamin Thompson, by then a colonel in the British Army, left England to seek his fortune on the Continent. He made his way to Bavaria where he became the confidential advisor to the ruler of that German state. It was in Bavaria that he conducted his most interesting investigations on heat. In 1792, the Elector rewarded Thompson with the title of Count of the Holy Roman Empire for his many contributions to Bavarian society. He chose to be called Count Rumford after the original name of the town in New Hampshire where he had started his career. In 1798, Count Rumford returned to England and immediately began work on his plans to create an institution for making the discoveries of science widely available through public displays, demonstrations, and lectures as well as formal instruction in the latest inventions and technical practices. In 1799, he founded the Royal Institution. It became an important center for the development of new scientific knowledge thanks to the discoveries of Humphry Davy and Michael Faraday. By inviting scientists and engineers from all over the world to give public lectures, the Royal Institution served as a place for the exchange and dissemination of scientific knowledge. Despite his shady past, Count Rumford's scientific investigations into the phenomena of heat were groundbreaking. Evidently, good moral character is not a prerequisite for developing correct scientific theories. |
|
|
If caloric is a "thing", it
must alter the weight of matter
it occupies.
But in his experiments, Rumford observed:
|
|
|
If caloric is a "thing," it must be finite in quantity.
But in his experiments, Rumford observed: It is possible to extract a seemingly infinite amount of heat from a cannon being bored by friction -- therefore, heat cannot be a "thing". |
| Rumford's findings weakened but did not break the hold of caloric on
the scientific imagination.
If Rumford is right and heat is the result of "motion" -- -what is moving? |
|