

Department of History
University of California, Irvine
Instructor: Dr. Barbara J. Becker
![]()
Week 7. The New Astronomy
excerpts from
"Spectroscopy and the Rise of Astrophysics"
(2003)
by Barbara J. Becker
in Storia della Scienza, Vol. VII: L'Ottocento
S. Petruccioli,
ed., Instituto della Enciclopedia Italiana
![]()
In her 1885 history of nineteenth century astronomy, Agnes Mary Clerke (1842-1907) enumerated the discoveries that marked the recent progress in that science. She drew her readers' attention to the founding of what she called "astronomical or cosmical physics", a new science that was markedly different in goals as well as methods from its older mathematical cousin. "It is full of the audacities, the inconsistencies, the imperfections, the possibilities of youth", she wrote. "It promises everything; it has already performed much; it will doubtless perform much more". [Agnes Clerke, A Popular History of Astronomy during the Nineteenth Century, Edinburgh 1885, pp. 183-184.] Clerke was not alone in her enthusiasm. Participants and observers alike perceived the development of this hybrid discipline as emblematic of the emergence of an era of productive cooperation among physical scientists linked by the spectroscope: a simple instrument consisting principally of a prism to disperse the light from a targeted source, and a sighting telescope to observe the details of the resulting spectral display. For years, the spectroscope had served physicists, chemists, and instrument makers as they probed the physical properties of light and the chemical properties of terrestrial materials. In the 1860s, they were joined by another, more eclectic group comprised in large part of amateur astronomers. These newcomers to spectroscopy were attracted by the instrument's potential as a tool for analyzing the light of celestial bodies. With it, they aimed to uncover the stuff and structure of the stars, a line of inquiry long-considered beyond the scope of legitimate science. Coupling the spectroscope to the astronomical telescope amalgamated the methods, instruments, and theories of chemistry and physics to those of astronomy suggesting new questions which, in turn, generated new mensurational tasks and ultimately realigned the familiar boundaries of acceptable research in astronomy. Chemists, physicists, and astronomers alike appreciated the growing intimacy and necessary interdependency of their areas of expertise. By the turn of the twentieth century, this "new" astronomy boasted its own set of questions, its own instruments, its own jargon, its own journals, as well as its own standards for assessing technical expertise and interpretational competence.
Looking beyond the conventional history of the emergence of astrophysics
reveals much about the social, cultural, and intellectual factors that
catalyze and nurture the development of a new science specialty.
Instead of a neat sequence of benchmark discoveries flowing naturally from
the skillful application of the spectroscope to astronomical problems,
we find a dynamic and often uncertain process. Those whose lives
and careers were shaped by the new science confronted challenging programmatic
choices, not between clear alternatives, but rather among an often confusing
field of theoretical and experimental options. Before anyone could
even see these options, the scientific community as a whole, and astronomers
in particular, had to be prepared and acclimatized to expect that celestial
bodies--like those in the terrestrial realm--may manifest behaviors that
offer clues to their internal natures; to recognize that these clues may
not be visually sensible; and to accept that even nonvisible physical properties
of celestial bodies--like those in the terrestrial realm--can be rendered
visible and analyzed with reliability and certainty through instrumental
means. Restructuring the aims and practice of astronomy in these
ways required a hardy blend of calculated risk, negotiation, and persuasion
on the part of a critical mass of willing and capable pioneers.
With the longitude problem solved and the challenge of the planet Uranus's orbital perturbations yet to be appreciated, one celestial mechanician worried that "in astronomy, human ingenuity will, probably, in future, be able to accomplish little more than an improvement in the means of making observations, or in the analysis by which the rules of computation are investigated". [John Narrien, An Historical Account of the Origin and Progress of Astronomy, London 1833, p. 520.] But, in fact, epistemological restrictions like those expressed by Bessel and Comte did not constrain astronomers' investigative imagination. A rigorous program of positional astronomy could and did reveal new and important knowledge of the heavens. The successful determinations of solar and stellar parallax, for example, the discovery of Neptune, and the confirmation of the existence of an unseen companion to the star Sirius--to name a few of the more familiar discoveries of this period--held out the promise that more intensive and extensive studies of the stars and their mutual interactions would make it possible to measure their stellar masses, distances, intrinsic brightnesses, and, perhaps, even determine the how and the why of their distribution in space. The growing numbers of amateur astronomers, meanwhile, were no less serious about, or adept at, studying the sky than their professional colleagues, but they occupied a wider range of investigative niches. They were merchants, artisans, lawyers, clerics, physicians, and landed gentry. Many were largely untrained in mathematics and less interested in the quantitative details of celestial mechanics than they were in the more qualitative aspects of the celestial realm. They were dilettante chemists, photographers, microscopists, and electricians who saw and interpreted astronomical phenomena with compound eyes and brains. They were tinkerers who used celestial bodies as extreme test cases for the sensitivity or accuracy of their instruments. They were opportunists who sifted patiently and tirelessly through the heavenly haystack hoping to be the first to discover just one of the proverbial needles that lay hidden there.... While some mid-nineteenth century astronomers were occupied with sunspots, terrestrial magnetism, and photography, others studied variable stars, binary stars, novae, minor planets, comets, and lunar and planetary surfaces. The mingled yarn of astronomers' diverse research interests created the weft and texture of the tapestry that became astrophysics, but spectroscopy was its warp. Indeed, the first tenuous threads of this fabric had already been strung by the time Robert Boyle (1627-1691) declared the prism to be the "usefullest Instrument" for gaining insight into the fleeting array of colors generated when sunlight passes through it. [Robert Boyle, Experiments and Considerations Touching Colours, London 1670.] In search of greater detail in the spectrum he had projected onto a piece of white paper, Boyle scrutinized the colorful array with a microscope, but saw nothing he could not observe with his naked eye. Boyle's experiments on color coupled with his enticing speculations that "perhaps" the prism would prove helpful in resolving questions about the root cause of all instances when color is observed encouraged others to employ it in their own optical studies. Isaac Newton (1642-1727) was one such investigator. Motivated by a desire to rid telescope images of the extraneous colors that distort them, young Newton began a series of prism experiments to identify the cause of, and a cure for the "celebrated phaenomena of colours" in all refracting optical instruments. [Isaac Newton, A Letter of Mr. Isaac Newton ... containing his New Theory about Light and Colours, Philosophical Transactions of the Royal Society of London, LXXX, 1671/72, pp. 3075-3087.] His observations convinced him that the elongation of the solar spectrum is due to its being composed of a series of overlapping images of the sun, each of a slightly different color. This discovery suggested a plan by which he could reliably analyze the solar spectrum aided by Descartes' law of sines. Struck by the elegance with which the law of sines reduced the complexities of refraction to a simple mathematical representation, Newton applied it to the case of sunlight entering and emerging from a prism. His investigations led him to conclude that when a beam of white sunlight is refracted by a prism, it displays not one, but a range of refrangibilities, each associated with a different color. In Newton's view, these results demonstrated clearly and incontrovertibly that color is not, as others had suggested, a modification of white light resulting from its contact and interaction with matter. It is, he claimed, an immutable property inherent in the stuff of light itself.
When Newton's new theory on light and color appeared in print in 1672, it created quite a stir. Individuals (e.g., Robert Hooke, Christiaan Huygens, Francis [Hall] Linus) who had conducted their own optical investigations were of the opinion that Newton's observations could be explained with existing modification color theories. Their inability to verify Newton's claims led them to question the legitimacy of his method as much as his theory. They faulted the paucity of information he had provided about his experimental design and the prisms he had used. And they complained that Newton had idealized his observations to the point where his results were, for all practical purposes, unattainable by anyone else. Distressed by the ensuing controversy, Newton lapsed into self-imposed silence on the subject of light for over thirty years leaving his disciples and opponents to continue the discussion. The critical attention accorded Newton's theory of color did not resolve the debate, but it did introduce and familiarize experimental philosophers of all stripes with what would become the fundamental elements of the method of prismatic analysis: a small aperture to admit a sunbeam into a darkened room; a lens to focus the beam; a prism to disperse the concentrated beam; and a means to examine the resulting spectral display. Newton published Opticks, his formal treatise on light and color, in 1704. He concluded it with sixteen rhetorical queries, which he amended and revised in later editions. In the queries, he speculated that light is a heterogeneous mix of "small Bodies" that respond dynamically when exposed to special short-range optical forces and active agents: reflection, refraction, and diffraction result from the deflection of particles in a light ray as they move within range of such a force. [Isaac Newton, Opticks. New York 1979.] Dispersion is a natural consequence of the varied responses of materially different light particles to these forces. For Newton's disciples, the queries served as a provocative road map to guide their future research on these forces. For his opponents, they were simply a provocation. Either way, during the century following Newton's death, experimental philosophers added the analysis of dispersed light to the arsenal of experimental strategies with which they attacked their increasingly varied lines of optical inquiry: the search for an experiment to determine whether light is particulate or vibrational in nature; the derivation of a mathematical law of dispersion that would hold for all materials at all times; the discovery of light's role as an agent of change in material substances; and the acquisition of an understanding of the similarities and differences between light and the phenomena of heat, electricity, chemical activity, and magnetism. In time triangular prisms of crystal or glass would be transformed from novelties of visual wonder and amusement into tools of analysis. The post-Opticks period was marked by methodological and programmatic ferment. The optical inquiries of John Michell (1724-1793) are of special interest in the context of the development of astronomical spectroscopy because he was the first to propose using prismatic analysis of starlight to learn something of the physical constitution of a non-terrestrial light source. Michell reasoned that light particles should be slowed by the gravitational pull of bodies from which they emanate. Although a preliminary calculation convinced him that solar gravity would have a negligible effect on the speed of sunlight, he later came to realize that light particles leaving the surface of a far more massive star could be significantly slowed. As these slower particles of starlight encounter the short-range optical forces in a glass prism, he predicted they would be deflected more from their original direction of travel than similar, but faster moving, particles of sunlight. Should a measurable displacement of a star's spectrum be observed compared to that of the sun, the star's relative mass could be deduced. Observers failed to verify Michell's prediction, however, undermining confidence in the line of astronomical investigation he had proposed. Nevertheless, his ability to imagine such a diagnostic role for prismatic analysis hints at the range and depth of the method's investigative potential in the minds of late-eighteenth century optical researchers. In 1783, acquaintances urged German-born musician, telescope maker and amateur astronomer, William Herschel (1738-1822) to view starlight through a prism. He did not pursue this line of inquiry until 1798, however, at which time he attached a prism to the eye-piece of his telescope to compare the dispersed light of a few bright, differently colored stars. Herschel discerned differences in the intensity and proportional distribution of colors in these spectra. Although the nature and degree of the differences he observed supported his view of stars as "opaque, habitable, planetary globes" differing from planets only in their size and intrinsic luminosity, he did not publish the results of his prism-based telescopic observations until nearly two decades later. [William Herschel, Astronomical Observations Relating to the Sidereal Part of the Heavens, and its Connection with the Nebulous Part: Arranged for the Purpose of a Critical Examination, Philosophical Transactions of the Royal Society of London, CIV, 1814, pp. 248-284.] Ignorance of the physical mechanism governing the action of the short-range forces deemed responsible for refraction and dispersion inspired rather than hindered experimentalists. They saw these forces as analogous and possibly identical to chemical affinities and electrical attraction, the poorly understood agents of change in the material world that also seemed to operate over short distances between small particles. In 1800, William Herschel positioned a battery of thermometers in the path of a dispersed sunbeam. He found the pattern with which the intensity of thermal rays varied was similar to, but not coincident with the spectrum of visible light. In fact, the thermal spectrum extended considerably beyond the red end of the visible spectrum. Although Herschel was able to reflect and refract thermal rays, and although he found that some of them have the same index of refraction as light rays, he nonetheless concluded that the two kinds of rays represent very different active principles. Herschel's discovery of invisible rays beyond the red end of the visible spectrum was greeted with enthusiasm by the German physician, Johann Wilhelm Ritter (1776-1810). Ritter, like other Naturphilosophie adherents, espoused a belief in the underlying unity of natural forces, and in their polarity as the root cause of all change in both animate and inanimate bodies. After learning of Herschel's rays, Ritter set out to find their polar opposites: invisible rays beyond the violet end of the spectrum. Where Herschel had used thermometers to detect and measure the intensity of his thermal rays, Ritter turned to chemical action as an indicator. Years earlier, Carl Wilhelm Scheele (1742-1786) had found that exposing silver chloride to light chemically reduced the compound, a reaction that was most effective when rays of violet light were used. Ritter placed paper soaked with silver chloride in the path of a dispersed sunbeam and observed that, while the reducing action of the violet rays was considerable, it was even greater in the area of the paper immediately adjacent to and just beyond the violet end of the spectrum. Further investigation revealed a bi-modal chemical spectrum in which the reducing action of Ritter's (Desoxygeneität) rays diminished and finally disappeared midway in the visible spectrum. Herschel's rays, which Ritter found to have an oxidizing action (Oxygeneität), picked up and gradually increased in strength toward and beyond the red end of the spectrum. From these observations, Ritter concluded that unrefracted visible light was the product of the neutralizing action of these two extremes of chemical activity. The extension of the spectrum into regions beyond the red and violet underscored the limits of human sensory perception. As prismatic analysis attracted the attention of investigators anxious to discover the relationships among visible light, radiant heat, and actinic rays, it became increasingly obvious that modifications in experimental design would be necessary before any non-visible effects they produced could be observed, recorded, and analyzed. John Frederick William Herschel (1792-1871), son of the illustrious astronomer, had earned international renown both for his astronomical research and his accomplishments in the field of chemistry. These two divergent interests served him well as he explored the emerging art and science of photography in the 1830s. The possibility that changes in photo-sensitive chemicals could be elicited by the action of rays beyond the red end of the spectrum led Herschel to test the effects of light on various chemicals by casting the visible and invisible rays of refracted sunlight onto chemically-treated paper. In 1840, to replace the thermometer as a means of detecting and measuring the action of heat, and to isolate the effects of radiant heat from those caused by other heat-related processes, Herschel developed a method for producing what he called a "thermograph", a fugitive image that made it possible, albeit briefly, to examine the range of heat ray activity. The thermic spectrum that Herschel observed was not continuous, but rather a chain of bridged oval-shaped "spots". In 1842, the English-born American chemist, physiologist, and pioneer photographer, John William Draper (1811-1882) modified Herschel's experimental set up by inserting a narrow slit in the path of the sunlight. With this simple, but important, improvement Draper resolved Herschel's spots into a series of separate lines reminiscent of those Fraunhofer had observed in the visible solar spectrum. Further advances in heat measurement were achieved using instruments based on the principle of the thermocouple. Leopoldo Nobili (1784-1835) and Macedonio Melloni (1798-1854) perfected an ingenious heat registering instrument called a thermopile, designed to convert minute temperature differences into a detectable electric current. Placing a thermopile at the focus of a telescope, Melloni made the first sensible measurements of lunar heat. Following Melloni's example, others turned thermopile-fitted telescopes on the moon, not out of interest in it as a celestial body, but to use it as a test source to gauge the heat-registering sensitivity of their instruments. Although the law of sines gave investigators some measure of experimental control over the variables that influence refraction's behavior, refractive dispersion was more capricious, varying from prism to prism, and from color to color. In 1802, retired physician and independent experimentalist, William Hyde Wollaston (1766-1828), studied the refraction and dispersion of light by different materials. He introduced two minor, but ultimately critical, modifications to the basic design of his prism experiments. First, rather than use a small hole to admit light from a source, Wollaston used a narrow, elongated "crevice" as an aperture. Second, Wollaston did not project the spectrum onto a screen for viewing. Instead, he held the analyzing prism directly in front of his eye. Observing the solar spectrum in this way, Wollaston was surprised to find it was interrupted by distinct dark lines separating it into four colorful regions: red, yellow-green, blue, and violet. Further examination revealed a total of seven dark lines which he interpreted as marking the boundaries of the primary colors.
In 1814, Fraunhofer began a search for a more reliable and efficient method based on optical theory. To limit the variables in his investigation, he looked for a source of monochromatic light. Filtering sunlight through colored liquids and glass produced unsatisfactory results, but he met with more success examining the dispersed light emitted by colored flames. Although he was disappointed to find that each colored flame produced a spectrum composed of a broad range of colors rather than the monochromatic signature he needed, Fraunhofer's attention was drawn to a bright and sharply defined orange streak that appeared to be common to all flame spectra. He searched sunlight for a similar bright streak, but found instead a closely-spaced pair of dark lines in that region of the solar spectrum. Closer inspection revealed the solar spectrum to be interrupted by "innumerable" (unzählig) dark lines. [Joseph von Fraunhofer, Bestimmung des Brechungs- und Farbenzerstreutuungs-Vermögens verschiedener Glassarten, in Bezug auf die Vervollkommung achromatischer Fernröhre, Denkschriften der Königlichen Akademie der Wissenschaften zu München, V, 1814-15, pp. 193-226.] He introduced a modification of the basic instrumental configuration used in prismatic analysis that would have far-reaching implications for future research in the field. Instead of observing the spectrum directly with his eye, Fraunhofer positioned a theodolite in the path of the refracted beam, thus creating an observing and measuring instrument that was the prototype of the modern spectroscope. With this instrument, he counted hundreds of dark lines in the solar spectrum and mapped the relative positions of many with precision. He developed an alphabetic system for labeling selected reference lines, assigning A to a line near the red end of the spectrum, D to the pair of dark lines associated with the bright orange streak he had observed in flame spectra, H to a line near the end of the visible violet, and I to a line in the ultraviolet. |
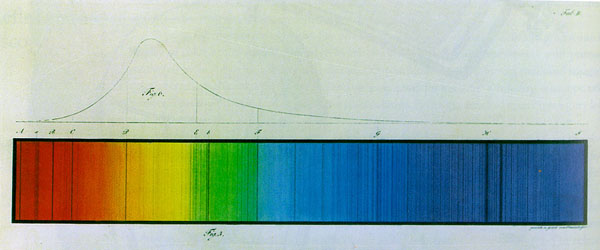
| Like Wollaston, Fraunhofer found a narrow slit to be the best aperture through which to view an extended light source, but he was concerned that diffraction effects at the edge of the aperture might influence the appearance of the spectral lines. In search of a bright and steady point source of light, Fraunhofer turned his spectroscope on Venus and several bright stars, not out of any astronomical interest in these bodies, but to have the opportunity to examine diffraction-free spectra. He found it impossible to discern the spectral characteristics of these dispersed point sources of light. To elongate the celestial body's light along the axis perpendicular to the prism's dispersive action, Fraunhofer added a cylindrical lens to the optical system. He noted that the spectrum of Venus contained lines identical to those in sunlight, but that stellar spectra exhibited greater variation. In fact, Fraunhofer conjectured that each star's spectrum was unique. Shortly after French physicist, Augustin Jean Fresnel (1788-1827) publicized his pioneering work on diffraction, Fraunhofer undertook quantitative studies of diffraction's dispersive behavior. He found that the spread of the resulting spectrum was inversely related to the width of the slit used to produce it. More importantly, he designed and made several diffraction gratings, first by winding wire neatly and tautly around a ruled frame, and later by etching parallel lines on a glass plate covered with gold foil to create hundreds of narrow, evenly-spaced slits. Finally, guided by the mathematics of optical interference and armed with diamond-ruled gratings boasting thousands of lines to the inch, Fraunhofer was able to calculate individual wavelengths of spectral lines. Though the normalized distribution of colors in diffraction spectra gave them a clear advantage over those produced by refraction, it would be several decades before diffraction gratings became widely incorporated in spectroscope design. Indeed, diffraction gratings were rarities until the late 1860s. In the meantime, the few gratings available were those ruled by Friedrich A. Nobert of Pomerania, who used his own closely guarded method to produce gratings with 100,000 lines per inch. Fraunhofer's lines puzzled practitioners and theorists alike for over four decades after the publication of his spectral maps. Although investigators attributed the dark interruptions in the solar spectrum to the action of some kind of absorption process, there was little agreement regarding the identity of the absorbing agent, and no physical theory to explain the why and how of its narrow selectivity. Convinced that optical phenomena and physical chemistry are intimately related, and optimistic that the key to their relationship lay in sorting the vast array of spectral line patterns, experimentalists, including David Brewster (1781-1868), William Henry Fox Talbot (1800-1877), Charles Wheatstone (1802-1875), and William Allen Miller (1817-1870), aimed to convert the muddle of empirical results into something resembling a coherent explanation of the appearance and probable cause of the lines by hunting monochromatic light sources, examining the solar spectrum transmitted through colored glass and through vapors, and investigating the flame, arc, and spark spectra of hundreds of substances. But their modes of attack and interpretive frameworks were wide-ranging, unrelated, and generally inconclusive. Investigators remained frustrated in their plans to identify a general physical or chemical principle of spectrum analysis until they were able to obtain reproducible evidence that a given pattern in terrestrial spectra uniquely signaled the presence of a particular substance. Furthermore, before theorists could formulate such a principle, and before practitioners could derive physical meaning from their spectroscopic observations, they had to recognize and demonstrate with certainty the direct correspondence between the patterns in terrestrial spectra and those of Fraunhofer's lines. Despite all their diligence and care, however, investigators were stymied by the ubiquity of Fraunhofer's D lines, the complexity of spectral signatures and the puzzling observation that some samples produced both emission and absorption lines. The critical insight that helped reduce the confusion came in 1857 as a result of a diversionary inquiry by Scottish professor William Swan (1818-1894). Hoping to reveal the mechanism by which light other than that generated by the sun was produced, Swan observed the spectra of hydrocarbons placed in the colorless flame produced by means of a new laboratory burner devised by the German chemist, Robert Bunsen (1811-1899) and his student Henry Enfield Roscoe (1833-1915). In the course of his investigation, Swan became intrigued by his observation that the outer portion of the lamp's flame flickered colorfully when anything, even the smallest dust specks passed through it. He wondered just how small an amount of a substance was required to create a colorful spectral display. To begin his investigation, Swan dissolved a small amount of common table salt in a large quantity of water. To his amazement, he found that less than one-millionth of a grain of salt was capable of coloring the flame with a bright yellow light. Clearly, spectrum analysis was a far more sensitive method than anyone had imagined. Swan warned chemists wishing to employ spectroscopy as an analytical method, to ensure that the sample to be analyzed contains no foreign material before drawing any conclusions about its chemical constitution.
On 20 October 1859, Kirchhoff presented a paper to the Berlin Academy on the Fraunhofer lines in which he offered both a chemical interpretation and a hint of a physical explanation to account for them. News of Kirchhoff's claim spread quickly throughout the scientific world via professional journals, personal correspondence and word-of-mouth. In fact, by October 1860 one would have been hard-pressed to identify a physicist, chemist, or optical instrument maker who had not heard something about Kirchhoff's discovery. That Kirchhoff observed the behavior he described was not disputed, but questions were raised concerning his ability to claim he had determined a physical cause for the appearance of the Fraunhofer lines. Some still wondered if the lines were caused by absorption of sunlight by the earth's atmosphere. Others complained that insufficient study had been made of the spectra of known terrestrial elements to draw any sensible conclusions from an examination of solar absorption lines. Despite these reservations, the absorptive and emissive behavior Kirchhoff had observed in the spectra of luminous gases seemed to many individuals so neat, so law-like, that they were willing to accept empirical evidence strongly suggestive of a physical connection between the spectra of metals and the Fraunhofer lines in the solar spectrum in lieu of an explanation. The spectroscopic discovery of three new elements--cesium, rubidium, and thallium--significantly heightened interest, particularly among chemists, in the empirical value of spectrum analysis to produce new knowledge. The hue and cry in popular lectures and journal articles about the validity of Kirchhoff's explanation for the Fraunhofer lines kept Kirchhoff's theory before the public eye long enough to be assimilated into a wider investigative context. In 1861, the English chemist, Henry Roscoe spoke enthusiastically about a "new stellar chemistry", that "now affords us certain information respecting the chemical constitution of the sun and the far distant fixed stars". [Henry Roscoe, A Course of Three Lectures on Spectrum Analysis, Lecture III, Chemical News, V, 1862, pp. 287-293.] A few years later, the German astronomer, Johann Karl Friederich Zöllner (1834-1882) spoke of "astrophysics" which he hailed as the union of "physics and chemistry with astronomy", an important first step in joining these "distinct disciplines into a higher and more general unit". [Hufbauer, p. 64.] Despite some reservations about what the pursuit of such research would ultimately teach investigators, chemist W. A. Miller, acknowledged that Kirchhoff's interpretation of spectral patterns could provide a "further glimpse into the machinery of the universe". [William Allen Miller, On Spectrum Analysis, Pharmaceutical Journal, Second Series, III, 1862, pp. 399-412.] It was tantalizing to think that interpretation of spectral signatures could bring human understanding of the chemical composition of the sun and distant stars to the same level of certainty as that attained by celestial mechanics. Where celestial mechanicians had founded the rigor of their science on painstaking observations of changes in position, celestial spectroscopists looked to indications of change in celestial spectra for clues to the correct sequencing of their evolutionary development. The multitude of variations in terrestrial spectra could be organized quite reasonably in a number of ways. Similarly, celestial spectra displayed patterns which mimicked distinctive features of their terrestrial cousins. All this gave rise to a dynamic interplay of observation, interpretation, and explanation among those working to establish celestial spectroscopy on a sound theoretical footing. There was little consensus among physical and chemical theorists over what it was, exactly, that the spectroscopic signatures of individual celestial bodies actually meant. That did not stop the speculation, it only encouraged it. Indeed, celestial spectroscopy soon thrived on the productive exchange of laboratory and field observations it generated. It provoked and sustained the discussion, comparison, criticism, and controversy necessary to develop confidence in the interpretive power of spectrum analysis as applied to the light of celestial bodies. Does each star possess its own individual spectrum? Are stars, as some stellar spectra seemed to indicate, composed of common terrestrial elements? Giovanni Battista Donati (1826-1873) was among the first astronomers to undertake a spectroscopic study of stars. In 1860, he examined the spectra of 15 of the brighter stars. Grouping these few spectra by their stars' visual color, Donati was struck by the "family likeness" of the spectral patterns in each color category. Although Donati's study drew criticism from his colleagues for the small size of his sample, it nonetheless inspired others to launch their own investigations and alerted them to potential observational challenges. Father Pietro Angelo Secchi (1818-1878), director of the Observatory in the Collegio Romano was one such motivated individual. Secchi had already shown his interest in spectroscopy years earlier by repeating some of Fraunhofer's observations, but his systematic spectroscopic examination of stars began only after he learned of Donati's efforts. Rather than subject a selected list of representative stars to careful examination, Secchi chose to launch a study of as many stars as possible. He assumed that spectral characteristics carried information about the physical properties of the light source, and that his spectroscopic survey would, therefore, provide clues to the nature of stellar atmospheres as well as help answer larger questions about the structure of the universe and the motion of stars. Over the years, Secchi catalogued the spectra of more than four thousand stars from which he developed a model star classification scheme based on spectral line structure rather than star color. The more stellar spectra he observed, the more sophisticated his classification scheme became. Starting with a simple two-category system, Secchi gradually distinguished three, then four spectral types. He supplemented his observations of stellar spectra with data he had gathered earlier on solar temperature, and with the results of new investigations he conducted on the effect of temperature change on the appearance of laboratory spectra. Based on these investigations, Secchi formulated a rudimentary stellar temperature sequence built around these four basic types of stars. In his classification scheme, the hottest, Type I stars, exhibit marked hydrogen lines in their spectra, while the spectra of Type II stars contain numerous fine dark lines. Type III stars have spectra that are divided by systems of nebulous bands that are more clearly defined towards the violet end of the spectrum. Type IV, the coolest stars, have spectra similar to Type III's but are more clearly defined on the red end of the spectrum. Secchi's confidence in the value and correctness of his classification scheme was bolstered by the ease with which so many stars fit into these four basic categories and the fact that stars of a given class seemed to be confined to distinct regions of space relative to the plane of the Milky Way. Another pioneer in stellar spectroscopy was Lewis Morris Rutherfurd (1816-1892), an American amateur scientist and instrument maker. With no spectroscopes commercially available when he first learned about Kirchhoff's work on spectrum analysis in the fall of 1861, Rutherfurd had to construct his own instrument before beginning his research. Unaware of Secchi's contemporaneous efforts, Rutherfurd observed the spectra of 23 stars. He was impressed by the variety in the number and positions of the absorption lines they displayed. Despite these differences, Rutherfurd defined three broad categories of stellar spectra based on a scheme quite similar to Secchi's: stars that were similar to the sun in color as well as number and position of absorption lines; stars with spectra similar to that of the star Sirius; and stars that showed no absorption lines at all. But Rutherfurd was driven less by an interest in the objects he observed than by a passion for the refinement and improvement of observing and measuring instruments. Even before he had heard of Kirchhoff's work, he, like Fraunhofer, had used spectroscopy to test the achromaticity of lenses so that he could pursue his interest in celestial photography. His concern for instrumental precision led him to design and construct improved micrometers, photographic equipment, and spectroscopes. A serious impediment to further standardization of spectral line maps lay in the spectroscope's central component: the prism. Although a prism's ability to disperse light is what gives it its unique analytical utility, recall that the optical principle behind the colorful display--refraction--is not a linear physical process. It is nearly impossible for different observers using different prisms under different circumstances to make direct comparisons of their results. Recall also that Fraunhofer had demonstrated how to eliminate this difficulty by replacing the prism with a diffraction grating as the dispersing element in a spectroscope. Because diffraction gratings were expensive and difficult to obtain, Rutherfurd began experimenting with ruling engines as early as 1863. In less than a decade, he was capable of producing gratings of higher and more consistent quality than those currently available. He generously made these gratings available to all who could make use of them. Indeed, it could be said that Rutherfurd's greatest contribution to astronomical spectroscopy was his work on diffraction gratings and his active promotion of their use in stellar spectroscopes.
Major alterations to the traditional organization of observatory work and workspace were required in response to the demands made by the intrusion of the new instruments and research goals. Huggins was key in laying the groundwork for the successful transplantation of the spectroscope from the chemist's laboratory into the domain of the astronomer. With Miller, Huggins arranged a "form of apparatus" capable of bringing the prominent spectral lines of the brighter stars into view so they could verify the earlier work of Fraunhofer and Donati. Although the two collaborators provide no details of their instrumental set up, it is likely to have been a makeshift arrangement which provided them with preliminary qualitative impressions. But the goal of their research was far more ambitious. The two aimed "to ascertain, if possible, the constituent elements of the different stars", and together, they expended considerable time and energy perfecting an apparatus capable of attachment to the observing end of a telescope with sufficient dispersion to permit rigorous comparison of stellar spectra to be made against that of the sun [William Huggins and William Allen Miller, Note on the Lines in the Spectra of some of the Fixed Stars, Proceedings of the Royal Society, XII, 1863, 444-445.] Huggins and Miller included schematic drawings of the absorption spectra of Sirius, Betelgeuse and Aldebaran in comparison with that of the sun. But they had not limited their efforts to these three stars alone. By the time Huggins and Miller completed their first round of investigation, they had surveyed the spectra of some fifty stars with this apparatus, as well as those of Mars, Jupiter and the moon, although only the spectra of Aldebaran and Betelgeuse were mapped to a level of completeness which satisfied them. Direct visual comparison of stellar spectra against those produced by known terrestrial elements was hindered by a lack of standard and precise spectrum maps. Huggins and Miller embarked on an extensive examination of metallic spectra, making significant improvements in instrument design and research methodology. Huggins devised an instrument with a graduated circle about which the viewing telescope could be moved and aligned with individual spectral lines using finely calibrated micrometer screws. He chose the interval between Fraunhofer's D lines as the standard of comparison thus making it possible to calibrate measures made by different observers using different prism-based instruments. Huggins' modifications shifted the mensurational focus of the spectroscopic investigation of starlight away from the sun and into the laboratory by enabling astronomers to rely on chemical spectra as standards of comparison, thus freeing them to conduct their investigations without having to track the sun or await its appearance. In 1864, Huggins' attention turned from analyzing stellar spectra to unraveling what he would later call the "riddle of the nebulae". [W. Huggins, The New Astronomy: A Personal Retrospect, The Nineteenth Century, XLI, 1897, pp. 907-929.] It was a bold move which ultimately propelled him to a position of prestige and authority among fellow astronomers. Nebulae are, as a class, among the faintest objects on the sky. Despite decades of telescopic observation, many questions concerning the physical nature of these celestial objects remained unanswered. William Herschel had defined a set of distinctive categories based on morphological differences which he then connected in temporal sequence. The resolution of some nebulae into star clusters by subsequent observers using larger telescopes than Herschel's raised the question of whether his so-called "shining fluid"--a sort of celestial humus which nourished the natural cycle of growth and decay in the heavens--was merely a chimera generated by the inadequate resolving power of available instruments. Based on an exhaustive examination of numerous nebulae with his unparalleled six-foot reflector, William Parsons, the third earl of Rosse (1800-1867), confidently declared many of them to be aggregates of closely-spaced stars. But, he confessed, his observations had only served to make the matter "more mysterious and more inapproachable". [Lord Rosse (William Parsons), Observations on the Nebulae, Philosophical Transactions of the Royal Society of London, CXL, 1850, pp. 499-514.]. It seemed the "riddle of the nebulae" could remain unanswerable in any positive, testable way.
The new field spawned by the introduction of the spectroscope into astronomical research developed rapidly and in a wide range of directions. No one knew which would prove most fruitful. In fact, Huggins' response provides insight into how patterns of individual choices add up to a major shift in the theory and practice of a science. Rather than take on the arduous task of systematically cataloguing the spectra of northern hemisphere stars, Huggins explored a number of different subjects in innovative and often technically challenging ways.
The spectroscopic study of novae is a case in point. The inability to impose artificial modifications on celestial objects and thereby "experiment" on them in the true sense of the word encouraged astronomers to pay special attention to those celestial objects which exhibited changes on their own. During the nineteenth century, there was an increase in interest among astronomers in noting and measuring variability in celestial objects. Thus, when a new star appeared in the constellation Corona Borealis (T Coronae) in May 1866, Huggins and Miller were the first to analyze its spectrum. They found the nova's spectrum to be compound, that is, comprised of a series of bright lines superimposed on a virtually continuous background. And, they correlated their visual observations of the nova's sudden rise and rapid decline in luminosity with concurrent changes in its spectrum. Huggins developed his own theory to explain its unusual appearance. His interpretive framework was guided, indeed limited, to the match of the nova's spectral line patterns with their artificially produced terrestrial counterparts: attributing bright emission lines to hot luminous gases and dark absorption lines to the passage of white light through a cooler vapor. He concluded that this star, by virtue of some cataclysmic event, had let loose a large quantity of hydrogen gas into the region immediately surrounding it. In his view, the intense heat of the star ignited the gas which was then consumed in a short period of time. Dubbing the nova a "star on fire", Huggins pointed out that he was only able to propose a physical explanation for the nova's change in brightness because of his careful spectroscopic examination of the star's light. He posed the provocative question of what the star's spectrum might have been like just before the outburst occurred. What about the bright lines seen in other stars? Could such a feature portend a similar cataclysm in these stars sometime in the near future? Huggins believed that proper interpretation of the differences in stellar spectral signature should lead to an understanding of the physical causes of variation in stellar luminosity. If a non-varying star with bright lines in its spectrum were observed methodically over time, perhaps a larger chain of events could be identified that ties nebulae, novae, and stars together in a progressive and physically justifiable sequence. When T Coronae was lost in the western twilight, Huggins moved on to such disparate projects as devising a method to observe solar prominences without an eclipse, spectroscopically determining the chemical composition of meteors, and visually corroborating reported changes in lunar surface features. It was during this period that Huggins made a brief foray into thermometric research. Recall that earlier work had focused on the nature of radiant heat and tests of instrumental sensitivity. After Kirchhoff's announcement, however, thermometric investigation became recognized as a means of obtaining information about the physical and chemical processes in celestial bodies. William Huggins was the first to try measuring the heat of stars. Convinced that heat from stars could be detected more easily than that from the moon, he believed reliable quantitative measures of stellar heat could be used to complement spectral data in the important task of determining the "condition of matter from which the light was emitted in different stars". He took special care to insulate the thermopile from all sources of extraneous heat, enclosing it within two tubes filled with cotton. To reduce the chance of conductive heat transfer, he separated the entire apparatus from the telescope body with wood. Beginning in the winter of 1866, Huggins observed at least five bright stars and the moon. He worked hard to cajole consistent results from his apparatus, but with little success. He conjectured that he would need a larger telescope to concentrate sufficiently the feeble stellar radiation. His disappointment over the unreliability of his results, coupled with the difficulty in converting deflections of the galvanometer's needle into an equivalent quantity of heat persuaded him to abandon thermometrics in favor of other projects. Huggins contemporaries were unaware of his efforts in this area, however, until the work of others urged him into print. It is, therefore, not surprising that Huggins' thermometric research has been ignored by his biographers and by historians of astronomy. Huggins is renowned for his innovative development of a spectroscopic method for determining a star's motion in the line of sight. Astronomers had long accepted that stars move in relation to one another, but it was only possible to measure a star's motion across the field of view, its "proper motion". Normal visual cues for judging a body's motion towards or away from the earth, its "radial velocity", are absent in the case of stars. Huggins believed that spectroscopy could be used to measure a star's motion along the line of sight by observing a selected line in a star's spectrum simultaneously alongside its counterpart produced by a known terrestrial element. Huggins reasoned that, like the change in pitch Christian Doppler (1803-1853) had correctly predicted would be heard emanating from a moving sound source, any lack of coincidence in the spectral lines' positions would be due to a shift in the wavelengths of starlight resulting from the star's motion relative to an earthbound observer. In February 1868, he began a series of visual observations comparing the hydrogen F line in the spectrum of a Canis Majoris (Sirius) to that of a laboratory hydrogen spark. To improve his chances for success, he acquired a more dispersive train of prisms and designed a new arrangement for throwing the comparison spark into the telescope that made it easier to align and compare its spectrum with that of the target star. Despite the inconsistencies in some measures, he concluded that the star Sirius was moving away from the earth at a speed of nearly 50 km/s. (According to modern measures, Sirius' radial velocity is 8 km/s toward the earth.) Huggins announced his results in tones of confidence and spirited adventure. Indeed, it could be said that his greatest contribution to the successful introduction of this new method into astronomical research lay in his ability to persuade his contemporaries that he had, in fact, accomplished what he claimed despite the overwhelming mensurational and interpretive difficulties the method entailed. Even though few understood the physical theory on which his line-of-sight measures were based, and the fact that implementing his method was largely beyond the resources and ability of many of his fellow amateurs, celestial mechanicians like those at Greenwich Observatory recognized its potential as an aid to their mission of charting the positions and motions of celestial bodies. The shifts observed in stellar spectra compared to laboratory referents were extremely small. A large part of the difficulty in implementing the method stemmed from the challenge of detecting these shifts through direct visual observation. At the time, the limitations in human visual response were deemed less of an obstacle than those inherent in existing photographic techniques. Fortunately, in the decades following Huggins' original announcement, advances in photography made it possible to record images of celestial spectra with sufficient definition that observers were able to measure the positions of spectral lines reliably. Two notable research programmes did much to reveal the tremendous analytic and interpretive power afforded by radial velocity measures. In 1886, Edward Charles Pickering (1846-1919), director of the Harvard College Observatory inaugurated the monumental spectrographic survey on which the Draper Catalogue of Stellar Spectra is based. And, in 1887, Hermann Carl Vogel (1841-1907), by then director of the Potsdam Astrophysical Observatory, and Julius Scheiner (1858-1913) launched an intensive photographic study of stellar radial velocities. Aside from providing celestial mechanicians with a dynamic, three-dimensional picture of the form and structure of the sidereal system, these surveys led to the surprising discovery that some stars which appear solitary to the visual observer, are, in fact, unresolvable pairs or groups of stars. Just as the spectroscope had generated new knowledge when first applied to the heavens in the 1860s, the spectrograph pointed astronomers in fruitful and previously unimagined investigative directions. Throughout the last quarter of the nineteenth century, spectroscopy was increasingly relied upon to analyze the light of comets, meteors, planetary atmospheres, solar prominences, and even the solar corona. As the art and science of chemical and astronomical spectroscopy became more refined, interpretive disagreements naturally increased. The spectrograph would prove essential in resolving these controversies. In 1888, William Huggins, working in collaboration with his wife, the former Margaret Lindsay Murray (1848-1916), focused his attention on the challenge of photographing the spectrum of the Orion Nebula (M42). He wished to determine the nature of the so-called chief nebular line, the green emission line that he had discovered years earlier in the spectra of planetary nebulae. Since its discovery, the chief nebular line had gained wide acceptance as a signature of certain classes of nebulae, but because it is located tantalizingly close to spectral lines associated with several terrestrial elements, attempts to explain the physical and chemical processes producing the line fueled a vigorous controversy.
Hoping to resolve this controversy in their favor, the Hugginses observed M42's spectrum between October 1888 and April 1889. These difficult observations required repeated direct comparison of the spectrum generated by burning magnesium against that produced by the faint nebula. To allow fatigued eyes to rest without interrupting the course of the evening's investigation, they alternated as observer and apparatus tender, their collaborative observations confirming their belief that the nebular line, though near that of magnesium, was nevertheless distinct from it. Convinced that visual observations alone would be insufficient to settle this question with certainty, the Hugginses overcame frustration and misadventure to obtain photographic evidence to support their claim. They presented what they and many of their colleagues viewed as a convincing argument against Lockyer's interpretation of the nebular spectrum. Although their sleuthing did much to cast doubt on incandescent meteorites as the source of the chief nebular line, the Hugginses could not establish "nebulum" as the only possible alternative. They, like all other empirical spectroscopists of their day, were limited to the familiar and, in most instances, adequately probative method of matching unknown spectra to those of known samples. With no physical theory to guide the development of alternative experimental strategies, the true explanation for the nebular line would remain a tantalizing mystery until 1927, when, in the wake of recent advancements in atomic theory, American physicist and astrophysicist, Ira Sprague Bowen (1898-1973) identified it as the mark of a so-called "forbidden" transition in doubly ionized oxygen (OIII), a physical process wholly unimagined--indeed, unimaginable--by pioneer spectroscopists like Huggins and Lockyer. |
Recall that William Huggins had subjected the light of a nova to spectroscopic study in 1866, and developed a theory to explain the event based on the unusual appearance of its spectrum. More than a quarter century later, in 1892, Huggins had the opportunity to examine the spectrum of yet another nova which appeared in January of that year in the constellation Auriga. Immediately after receiving word of the nova's appearance, Huggins and his wife joined astronomers around the world in scrutinizing the nova's light. Astronomers, physicists, and chemists had no better understanding of the physical mechanisms at work in such celestial events. They were still at work speculating on what causes spectral lines to appear in the first place, as well as what occasionally makes them disappear, widen, split into doublets, or shift. Still, this time the interpretive guidelines were richer. Huggins himself had linked the shift of spectral lines to relative motion of the light source along the line of sight. The gross features he had noted in the 1866 nova--namely the appearance of bright and dark lines--were universally observed in the spectrum of Nova Aurigae. But this time, the bright lines could be (and were) described as greatly widened and shifted toward the red end of the spectrum. Many of these bright lines were accompanied by adjoining absorption lines which were shifted toward the blue. The shifts observed could be (and were) interpreted as indicating that the light being analyzed was coming from at least two different sources--one a luminous gas or bright-line star moving away from Earth, the other a more sun-like object moving towards the earth--each at tremendous speeds. Some of the dark and bright lines were seen as doublets and possibly triplets, an observation which led to early speculation that this event involved the explosive collision of perhaps as many as six bodies. Huggins preferred to construct a scenario based on more familiar themes: The bright and dark spectral lines signifying, perhaps, the onset of violent stellar eruptions spewing out into space hot gaseous material that had been trapped under the cooling crust of an ancient star. Such eruptions would be similar to, but more extreme than, those seen almost daily on the sun. Alternatively, the lines could indicate the creation of multiple "reversing layers" due to turbulence in the normal structure of a star's atmosphere caused, perhaps, by the tidal forces of a nearby companion or passing star. Such multiple reversals, Huggins argued, had been artificially induced in terrestrial laboratories and could signal the existence of dynamic processes within stars. While such conditions would hardly be considered routine, he viewed them as more likely than having six stars independently moving toward each other on a collision course. The length of time that the nova's light remained sufficiently bright to analyze gave unprecedented opportunities for careful examination by the world's astronomers. William Wallace Campbell (1862-1938) of the Lick Observatory in California announced that recent photos of the nova showed it to be distinctly nebular in appearance. In fact, he claimed, subsequent examination of the nova's spectrum indicated the distinctive spectral signature of a planetary nebula. Huggins found it difficult to imagine that such rapid change in the nova or its spectrum could realistically occur. He blamed the Lick's blurry photographic image of the star on failure to focus its light properly for the range of wavelengths being emitted during its outburst. And he forcefully questioned Campbell's interpretation of the nova's spectrum. Indeed, when Nova Aurigae returned to a favorable position in the evening sky the following February, Huggins examined its now feeble light specifically for signs of nebular characteristics. He would know a planetary nebula spectrum if he saw one, and Nova Aurigae's spectrum, with its complex array of bright lines, did not display the features necessary to classify it as a planetary nebula. Instead, Huggins likened its spectrum to that of bLyrae, a star with a spectrum of bright and dark lines that vary in brightness, width, and structure in complex ways. Modern views support Campbell's interpretation of the evidence, but it is important to recognize that it was not the only one that was seriously considered on the evidence available when the nova first appeared. While Huggins' physical explanation for the events leading up to the nova's sudden outburst seems contrived to us today, from Huggins' perspective, it was Campbell's scheme that was hard to swallow if the rules of scientific explanation restricted legitimate evidentiary interpretation to that based on the simplest known mechanisms and the least speculation. Without the ability to modify artificially the conditions under which celestial bodies produce their light, eclectic and opportunistic observers like Huggins could only extrapolate from the laboratory to the field and back again by working fast and loose within an as yet incomplete and uncertain system of physical theory. It was a risky business. Nevertheless, by allowing a wide range of interpretive schemes to exist simultaneously and to interact fruitfully, the lack of clear explanatory guidelines in this fledgling discipline often proved to be a help rather than a hindrance for these audacious pioneers. For others, mainly institution-bound professional astronomers, the uncertainty of this pre-paradigm phase of astrophysical investigation demanded a more Baconian approach featuring systematic, large-scale surveys of stars, like those conducted in the 1860s by Father Secchi. Projects of that magnitude required the long-term financial support of a wealthy and reliable patron. Public institutions relied, for better or worse, on government funds, while private institutions courted the generosity of interested individuals. It was thanks to the gift of funds and instruments from Mary Anna Palmer Draper (1839-1914), widow of astronomer Henry Draper (1837-1882), that E. C. Pickering was able to undertake the ambitious task of photographing and cataloguing the spectra of all the brighter stars visible in the northern hemisphere. Such a task did not afford observers the luxury of devoting individual attention to each and every star. By dispensing with a slit, using a large prism in front of the telescope's objective lens, and allowing the earth's motion during a timed exposure to sufficiently widen each star's spectrum, Pickering's team was able to capture the spectra of all the stars within the telescope's field of view, sometimes as many as 200, in a single image. The first installment of the Draper Catalogue of Stellar Spectra, contained the spectra of over 10,000 stars. It was not merely a data table. From the beginning, the catalogue was intended to serve as a working stellar classification system: structured around a sequence of seventeen categories (A-Q) derived from Secchi's scheme, yet amenable to modification and improvement as additional spectra were recorded. Comprised of an extraordinarily large--and continuously growing--sample of stellar spectra, the Draper Catalogue was an ideal subject for the kind of rigorous statistical analysis that Pickering rightly anticipated would lead to new knowledge of the physical and chemical constitution of stars. In 1911, Annie Jump Cannon (1863-1941), then curator of astronomical photographs at the Harvard Observatory, began work on the creation of a new Draper Catalogue, classifying the spectra of over 5,000 new stars per month for four years. The resulting nine-volume catalogue of nearly a quarter-million stellar spectra was an unparalleled achievement. For the pioneers of astrophysics--whether independent investigators like Huggins or large-scale project directors like Pickering--the relationship between laboratory and field observations was a productive, if sometimes contentious symbiosis. The discussion, comparison, criticism and controversy it provoked built confidence in the interpretive power of empirical celestial spectroscopy. Contemporaneous developments in atomic theory enhanced the explanatory power of all spectral signatures. As the nineteenth century drew to a close, the questions astronomers could ask about the bodies they observed and the methods deemed appropriate to examine them had changed in ways their predecessors could hardly have imagined. A generation later, individuals choosing careers in astronomy could look forward to learning the nature of novae; the physical and chemical meaning of the range of appearances of stellar calcium lines; the source of variation in strength of hydrogen and helium lines in different stars; the causes of solar eruptions; the structure and substance of the corona; the structure of the Milky Way; the dynamics of stellar motions; and even the source of stellar luminosity itself. |
|