
University of California, Irvine
Instructor: Dr. Barbara J. Becker

|
Week 10. New Worldview X-rays and Radium in the News
|
|
New York Times
POWER OF ROENTGEN RAYS
Some Effects of the Discovery Studied
PRINCIPLE INVOLVED IN EXPERIMENTS
Impression of the Human Hand with
The following article, taken in part from the French journal l'Illustration, describes the effects of the Roentgen rays as seen by French observers, and also give an impression of a hand prepared by Voller of Hamburg made from the actual plate and wholly trustworthy in detail: Among the experiments made, that of the interposition of the hand seems to be one of the most fruitful. When the experimenter beheld the picture, or rather the shadow of the skeleton, appear upon the plate he found himself in the same position with Daguerre or Niepce when these sought to "fix" the picture in the parkroom--that is to photograph it. It was only a step then to ascertain whether the ordinary photographic plates were sensitive to the new rays. This sensitiveness having been found to exist, the results obtained could be illustrated, fixed in fact, in a startling manner. Photography through opaque bodies had been discovered! The principle is that shadows form regularly on the photographic plate as they do on the fluorescent surface whenever bodies of unequal transparency are placed between Crookes's apparatus and themselves. As the density diminishes, the transparence of the reliefs becomes more perceptible. By proceeding in the manner described M. Roentgen and other operators,
such as M. Voller (Hamburg) and Dr. Oudin (Paris) were able to obtain photographs
of human hands.
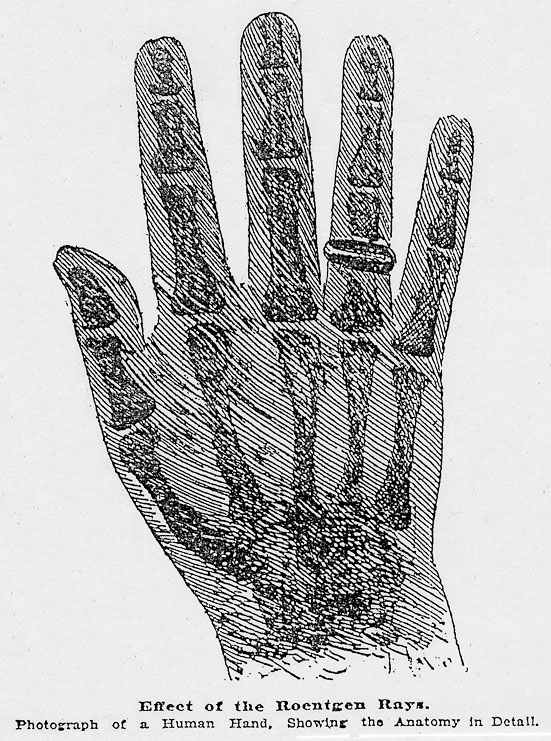
Effect of the Roentgen Rays.
Each detail of the anatomy is recorded there with marvelous fidelity, the bony matter intercepting the rays. The flesh, the muscles, the tendons, the veins and arteries, the dermis and epidermis, are only represented by a hazy shadow, light, undefined, scarcely perceptible, none of the tissues causing it being impenetrable to the new light. The thick metal ring projects a still darker, denser shadow than the bones. In the same way, scissors in their sheaths and bolts through their wooden doors have also been photographed. The photographs obtained in this way have nothing in common with ordinary photographs. They are not rays reflected by the objects whose picture it is desired to obtain, but rays emanating directly from the luminous tube. The shadow of the interposed object defines itself clearly upon the plate, blackened either by the direct action of the rays or by the fluorescence engendered in the glass. Differing from the photographic eye, the human eye seems to be insensible to the Roentgen rays, since, when the tube whence they proceed is inclosed in an envelope, it is not possible to perceive the slightest glow. Nevertheless, a French experimenter, who has reproduced in Paris the experiments of the Professor of Wurzberg, declares that a hand placed against a Crooke tube becomes itself fluorescent, and shows to the eye sufficiently educated its most minute anatomical structure. This would, indeed, realize the power of seeing through opaque bodies. But what then is this adaptation of the eye but which enables hypnotic subjects to see phantoms through brick walls? However this may be, the new rays discovered by M. Roentgen have as yet revealed, even to their discoverer, but little of their nature, and the first hypotheses yet remain to be verified. Is this discovery likely, from a practical point of view, to produce appreciable results? Can it be used in the world of medicine to determine the nature of an organic lesion, or the form of a tumor in the interior of the body? The Germans have already essayed experiments in this direction. A Berlin doctor this week took a photograph by means workingman wounded by an explosion of glass, and in the proof obtained could plainly be seen the pieces of glass sticking in the bone. In conclusion, as yet neither the laws nor the theory of these phenomena are known, but the phenomena themselves are sufficient to give us a glimpse into a new world. |
|
London Times
PROFESSOR CURIE ON RADIUM.
There was a very large audience at the Royal Institution last night to hear Professor Pierre Curie, of the Sorbonne, Paris, lecture on radium. Sir William Crookes was in the chair, and among those present were Mme. Curie, Lord Kelvin, Lord Rayleigh, Lord Avebury, Sir Frederick Bramwell, Sir Oliver Lodge, Professor Dewar, Professor Ray Lankester, Professor Ayrton, Professor S. P. Thompson, and Professor Armstrong. Professor Curie, who spoke in French, began with some experiments to illustrate the properties of radium. He showed that it was capable of spontaneously and continuously disengaging heat, that it had the power of rapidly affecting photographic plates even through opaque bodies, and that it could provoke luminous phenomena in phosphorescent substances such as platinocyanide of barium, not losing its power even when cooled to the temperature of liquid air. He next proved its ability to render air a conductor of electricity, by showing that a charged electroscope was at once discharged when a fragment of radium was brought into its vicinity, and in another experiment showed that it facilitated the passage of the electric spark. He went on to describe the different radiations given off by the substance and to distinguish them according to their power of penetration, absorptibility, behaviour in a magnetic field, &c. He then explained that, in addition to these radiations, radium also gave off emanations which had the same properties as the substance itself--properties which were included in the term radioactivity. The salts of radium in solution gave off this radioactivity, and were able to render other objects of all sorts radioactive. In this emanation, for example, a charged electroscope was discharged, and a phosphorescent substance became luminous. The emanation behaved in many ways like a gas. It could be aspirated through a tube, it could be condensed by liquid air, and after being frozen out of a vessel would diffuse throughout it again when the temperature was allowed to rise. These phenomena were illustrated by a very pretty experiment, in which a vessel containing a weak solution of radium chloride was connected by a tube to another vessel containing some sulphide of zinc. So long as the stopcock on the tube connecting the two vessels was closed the sulphide of zinc did not phosphoresce, but as soon as it was opened the luminous effect appeared. Returning to the heat disengaged by radium, the lecturer proved the reality of the phenomenon by the aid of what he said was in fact a liquid air calorimeter. A small piece of glass was lowered into a carefully isolated vacuum-flask containing liquid air, and the amount of gas that boiled off in a given time was measured. The experiment was then repeated, but instead of the plain piece of glass a small vessel, identical in size, containing radium was substituted, with the result that in the same time the quantity of gas given off was seen to be more than doubled. Professor Curie concluded with a slight reference to some other properties of radium, its chemical effects, its place in the periodic table of the elements, its power of producing sores on the skin and even of inducing paralysis, and the character of its spectrum. He also gave a brief account of the studies which led Mme. Curie and himself to the recognition of it and other radioactive bodies, and touched on the speculations suggested by the phenomena it presented as to the evolution of matter and the gradual transformation of the elements. |
|
London Times
THE MYSTERY OF RADIUM.
Last March The Times announced the discovery by M. Curie of the astonishing fact that Radium, in addition to the radio-active properties rendered more or less familiar by the researches of M. Becquerel on uranium, possesses the property of maintaining its temperature at a point three degrees higher than that of its surroundings, and of continuously emitting heat without any apparent diminution of bulk or alteration of physical constitution. This announcement was received with great incredulity. Eminent men of science refused to accept a statement so irreconcilable with scientific experience, and maintained that there must have been somewhere a serious error of observation. That Radium possessed radio-active properties indefinitely more powerful than those displayed by any other body was a fact of an order to which we were accustomed. Those properties, however remarkable, differed only in degree from properties with which the scientific world was familiar. That difference in degree has indeed become sufficiently astonishing in the light of further study, for it has become clear that Radium without external stimulus can produce effects hitherto only obtainable by means of the electric discharge in high vacua. It can throw gases into that state of vibration which causes the production of their characteristic spectrum, and it emits at the same time a radiation resembling the Röntgen rays and producing like them marked physical and physiological effects. Superadded to this extraordinary development of powers not unfamiliar in their lower manifestations, is the unique and unprecedented power of emission of heat, which is now established beyond all possibility of question. That gross physical effect, in addition to the radioactive and physiological effects produced on so large a scale, points to an amazing total output of energy for which no compensation has yet been discovered. Strenuous efforts have of course been made to obtain accurate measurements of the heat production, and to determine the effect of external conditions in promoting or retarding it. M. Curie has just communicated to the French Physical Society a paper stating the results of a recent inquiry. It appears that at the time of his lecture at the Royal Institution in June, the resources of that laboratory in producing and manipulating liquid gases were utilized in a new series of experiments. Professor Dewar had already in 1893 improved the calorimetric use of liquid gases by means of a combination of vacuum vessels so that heat-evolution at the temperature of boiling liquid air or hydrogen could be determined with accuracy. When a sample of Radium bromide weighing 0.7 gramme was tested in this way, it was found to be capable of volatizing an amount of liquid oxygen and hydrogen equivalent respectively to 6 c.c. and 73 c.c. of the gases measured at the ordinary temperature. It seems that through a very wide range of temperature the thermal emission remains unchanged. Whether at the temperature of a summer day or at that of liquid air, the emission of heat goes on without perceptible variation. When, however, we make a long downward stride from liquid air to liquid hydrogen, Radium shows that it is not always unaffected by external temperature. Within a comparatively short distance of the absolute zero a change occurs in the rate of heat-emission, but not in the direction that might be anticipated in view of the effect of low temperatures on ordinary chemical action. Instead of being reduced, the emission of heat, so far as present data can be relied on, is augmented at the temperature of liquid hydrogen. Whatever be the nature of this extraordinary phenomenon, it only increases in intensity at a point where all but the most powerful chemical affinities are in abeyance. The evaporation of a liquid gas gives an absolute measurement of the amount of heat given off by radium. Changes in the degree of radio-activity may escape the most careful observer, or may be imagined where they do not exist, but the quantity of liquid hydrogen which a given mass of Radium converts into gas in a given time can be easily measured with an accuracy which is beyond cavil, and the amount of heat required for the conversion can be ascertained with great precision. Hence there is no longer any doubt either of the quantity of heat evolved by Radium or of the fact that the rate of emission is apparently greater in liquid hydrogen than at any temperature from that of liquid air up to that of an ordinary room. At the beginning of these experiments in liquid hydrogen, a contrary result appeared to emerge when the low-temperature thermal measurements were compared with the early Curie values observed at the temperature of melting ice, as formerly given in The Times. This led to the curious discovery that a freshly prepared salt of Radium has comparatively feeble power of giving off heat at all temperatures, but that its power steadily increases with age until about a month from its preparation, when it reaches the maximum activity which it afterwards maintains apparently indefinitely. A solution of a Radium salt behaves in exactly the same way. Its power of heat-emission is at first relatively low, but goes on increasing for about a month, when it becomes equal to that of the solid salt, and so remains. These remarkable results throw no light upon the process by which Radium maintains its constant emission of heat and radio-activity; but they will have to be accounted for by any theory that may be constructed. |
|
New York Times
TO MAKE LUMINOUS
Drs. Kunz and Merton Discuss
Interiors of Patients May be Lighted
Radium and actinium were discussed last night before the Technology Club of New York in the operating rooms of Dr. William J. Morton, 10 East Twenty-eighth Street, by Dr. George F. Kunz and Dr. Morton, who is Professor of Electro-Therapeutics in the New York Post-Graduate Medical School and Hospital. Dr. Kunz reviewed the experiments which have been made with radium in Paris from a metallurgical point of view. He told of the many interesting qualities of pitchblende, taken from Pribam, in Austria, which he asserted, resembled radium in many respects. The doctor had with him a specimen of radium, and he also exhibited a sample of American radium ore which was discovered in Colorado. His exhibits also included a sample of actinium, which he said was the first specimen of that ore ever displayed in public. In order to carry out the experiments it was necessary to extinguish all the lights in the room. That being done Dr. Kunz took from his pocket a small piece of radium about the size of a pin head, which he said was of 100,000 activity, and had cost nearly $300. The radium was inclosed in a glass tube, outside of which there were four other tubes of copper, iron, rubber, and lead. He then produced the famous "Tiffany diamond," weighing more than 15 carats. In order to show the luminous properties of the radium he held the diamond about two inches away from the tubes, and exhibited it to the audience. The light seen was like that of phosphorus. It would have made no difference, the doctor said, if the tubes had been a foot thick, the glow would have been discernible. Afterward the diamond was placed in a glass of water, where it shone beautifully. The bombardment of radium was demonstrated by means of the spintharescope, and it looked like a tiny Roman candle throwing off a myriad of sparks. Dr. Kunz said he thought that in time radium of a very high activity would be obtainable, and he would not be surprised if it reached 1,500,000. Dr. Morton explained in detail the uses to which radium might be put in curing diseases, particularly those of an internal nature. "Medicine," he said, "is gradually abandoning its old-fashioned concoctions, and we are taking up radium with exceedingly bright prospects. Its use will consist of physical treatment almost exclusively. The Roentgen ray has been of immense value in curing cancer, but radium promises to go far ahead of it. If we had radium of 150,000 activity we could no doubt do a great deal more than we are doing now. Most of us have been confined to a much lower radio-activity. We have been working with from 7,000 to 10,000 luminosity. "The actual glow of radium does not represent its actual radio activity. There is a great difference in the ore. One sort of radium may possess a high luminosity, while another sort may have a high radio-activity and very little luminosity . We cannot boast of the luminosity of the kind which we now have." Dr. Morton startled his hearers by telling of a mixture which he had prepared and called "liquid sunshine," the name having been applied because the Doctor regarded it a good "catch" phrase to give to the preparation. By means of this fluid, he said, the whole interior of a patient could be lighted up. The doctor exhibited six tubes containing "liquid sunshine," one of which, he explained, contained quinine sulphate which had been exposed to radio activity. He then proceeded to show the luminous quality of the fluid by placing each tube before a strong X ray, whereupon a spot of faint light was seen abut the size of the palm of the human hand. "That," said the doctor, "would be the result if the liquid were taken inside. I believe," he added. "that radium may after all be the real curative property which has been found in so many spring waters throughout the world. "The advantage of radium over the X ray is that it can be applied direct to the part affected. For example, if placed in a small tube it may be inserted in the throat, and in similar manner it may be applied to any vital region. In other words, with radium we shall be able to get at the seat of diseases. There is no end, in my opinion, to the cures which may be effected by radio-activity, excited in one way or another. "In imparting radio-activity to liquids, however, we will have to be extremely careful, and physicians will need to use the utmost discretion in advising patients to drink the fluid. It will be possible, however, to bathe a patient's entire interior in violet or ultra violet light as the result of this discovery, and this light we have decided to call 'sunshine.' We know of the value of sunshine on the outside, particularly where bald heads are concerned, and we believe it will have a similar effect on the inside." Dr. Morton told of several cures of cancer by radium, and exhibited a bell-shaped glass, where the smaller tubes of radium, of about 7,000 activity, could be placed in the flesh affected. As the activities of radium became greater, he expected that more important results would follow. |
|