
"Madame Skladovska Curie," from A History of Chemistry, by F. G. Moore (1918), opposite p. 149. |
Teacher Notes on
an excerpt from the
WestEd KENDALL/HUNT PUBLISHING COMPANY |

"Madame Skladovska Curie," from A History of Chemistry, by F. G. Moore (1918), opposite p. 149. |
Teacher Notes on
an excerpt from the
WestEd KENDALL/HUNT PUBLISHING COMPANY |
Seated among such scientific greats as Albert Einstein, Niels Bohr, Enrico Fermi, and Ernest Rutherford in the classic photographs taken at the Solvay Congresses, Marie Curie's gaunt figure grabs the viewer's eye: it is a feminine form, seemingly forged from hard steel. Ever serious, brows furrowed, jaw set, eyes penetrating, Marie Curie has served as a role model for generations of young women who have been inspired by her perseverance and dedication to pursue a scientific career. Who better to tell the story of one of the most remarkable scientific adventures of our century than the person who started it all?
Margaret Lindsay Huggins is not a familiar name. Although she lacked the formal scientific training that Marie had received, Margaret had been a practicing scientist for nearly 30 years by 1903, working collaboratively with her husband, the astrophysicist, William Huggins. Always interested in new things to examine using spectroscopy, Margaret and William began work on the spectrum of radium immediately after the Curies' visit to London.
We don't know if these two women met during that visit, or even if a conversation of the type that has been created for the video drama actually took place. Still, circumstances were such that opportunities would have existed for Margaret and Marie to have exchanged more than a handshake. In 1903, William Huggins was the president of the Royal Society of London. He and Margaret hosted a party at the Royal Society in honor of the Curies--an occasion that was marked by a brief news article in the London Times. As a leader in London's scientific community, William Huggins would likely have had several social and professional meetings with the Curies during their stay. Given Margaret's scientific interests, it is hard to imagine that she would not have been anxious to join such meetings. Certainly, as wife of the Royal Society president, Margaret may have shepherded Marie around the sights of London, or simply shared an afternoon's chat with her in one of the city's lush green parks.
In this unit, there are a number of subtexts, the most obvious of which is that creative and curious women have made important contributions to science, even when circumstances didn't make it easy for them to do so. Another, perhaps more subtle message is that questions about the structure of matter were very hard to ask at the beginning. It wasn't clear whether the study of radioactive materials would ultimately point researchers in the right direction, or send them on a tedious dead-end journey. Radioactive phenomena had all the earmarks of something novel and intriguing, but many scientists felt that they would prove to be little more than idle amusements. Thus, Marie's rigorous pursuit of this line of investigation is a credit to her perseverance and resistance to conformity. A third message is that science is difficult to do alone. Collaboration is not only an efficient way to accomplish what needs to be done, it is often the only way it can be done. The best research teams are composed of individuals with complementary talents and skills. Teams that nurture the unique talents of their members will be the most successful.
![]()
1. PRE-ACTIVITY--RÖNTGEN'S INVISIBLE RAYS The essay, "Röntgen's Invisible Rays," is a motivating account of Wilhelm Röntgen's discovery of X-rays. It is intended both to pique your students' interest in the study of radioactivity, and to familiarize them with the scientific and historical background that underpins the "Atoms and Matter" unit. Benchmark issues:
|
Although "Röntgen's Invisible Rays" is intended as a homework reading assignment, you may wish to develop a more elaborate in-class activity around it, particularly if you normally include discussion of X-rays in your instructional program.
It is difficult to grasp the excited amazement that erupted worldwide immediately following Wilhelm Röntgen's (pronounced, VIL-helm REHNT-ghen; 1845-1923) announcement of his discovery of X-rays. Having an X-ray is a routine medical procedure nowadays. Comic book heroes are born with special X-ray sensors. Airports have added X-rays to their arsenal of security measures. But when news of Röntgen's discovery first broke in January 1896, the story made front page headlines around the world.
Images produced by X-ray action were initially called shadowgraphs because they are silhouettes of objects that are opaque to the penetrating X-rays. X-rays could reveal the shapes of objects that are normally hidden from view, like the bones in a living human hand. The potential benefit of the penetrating rays to medicine was immediately recognized. For investigators in the physical sciences, Röntgen's remarkable discovery launched a wholly new and productive line of inquiry into the structure of matter. Everyone had questions about the cause, physical nature, and utility of the mysterious rays. No one had any answers.
If you assign "Röntgen's Invisible Rays" as homework reading, alert students that there will be time for sharing their thoughts about the story at the beginning of the next class meeting. If your students read this essay during class time, you will want to allow time for discussion. (See sample questions in Lesson Plan One--Opener for discussion guidance.)
In addition, two related supplementary items are included here and in the Student Reader. The first is an article from the New York Times ("Power of Roentgen's Rays," February 4, 1896) announcing Röntgen's discovery. Students can compare Röntgen's X-ray photographs of the hands of Frau Röntgen and Professor von Kolliker with the newspaper's drawing of a similar X-ray photograph taken by Monsieur Voller of Hamburg.
The second is excerpted from "The New Marvel in Photography," which records a British journalist's exclusive interview with Röntgen. What if you were a journalist and your boss ordered you to interview a scientist who had just made an important discovery? How would you contact the scientist? How would you convince the scientist to take the time to answer your questions? What would you ask? What would you expect to see? What would you tell your readers about your experience? In the spring of 1896, as the world was just getting word about Wilhelm Röntgen's mysterious X-rays, a British journalist, H. J. W. Dam, was asked to do just that. But when he arrived in the town where Röntgen lived and worked, Dam discovered, much to his dismay, that Röntgen refused to talk with any reporters about his new X-rays. Unwilling to go away empty-handed, Dam expressed his irritation in a letter to Röntgen telling him that he was the most difficult of all the famous scientists he had ever interviewed! Röntgen was apparently so startled by the journalist's audacity, that he granted Dam the only personal interview he ever gave on the subject. The text of Dam's interview appeared as the lead article in the April issue of McClure's Magazine accompanied by numerous photographs showing the wonders of X-rays.
![]()
2. LESSON PLAN ONE--INTERPRETING SHADOWS a. Concept for the Day
Benchmark issues:
|
b. Opener
If your students have read "Röntgen's Invisible Rays" as a homework assignment, start out with a brief review of its contents and implications. Some sample questions are provided in the Opener of "Lesson Plan One" to guide your discussion.
NOTE: Keep in mind that the lesson's focus is not on X-rays themselves, but on their role as invisible messengers carrying information about the structure of the atom to those investigators patient enough to learn how to read them.
If your students read the essay as an in-class activity and you have already led them in a discussion of it, take a little time here to reinforce its key point, namely that Röntgen's remarkable discovery gave scientists a new way to examine things that are normally hidden from view and pointed them toward a wholly new and productive line of inquiry into the structure of matter.
To move your students from the abstract realm of indirect experience (reading or second-hand description, for example) to the concrete realm of direct experience (available only through active participation), provide them with a visual prompt. Project the image of an X-ray photograph (preferably one that shows an easily identifiable skeletal structure such as a hand, foot, or an arm) on a screen using an overhead projector.
NOTE: Obtain an old medical X-ray from a physician, veterinarian, or radiology lab. Be sure that any information identifying the individual in the image has been removed or covered before displaying it in your class.
Use the suggested guide questions provided in the lesson plan to reinforce your students' understanding that:
In the course of this discussion, emphasize the value of inference in scientific investigation, particularly for those investigations involving phenomena that are inaccessible using more direct observational techniques.
c. Activity 1--Whatzit?
Humans possess no natural ability to detect an individual atom. We cannot taste, touch, smell, see, or hear such things as radioactive decay. Nevertheless, scientists now know and understand a great deal about the way nature works on the atomic level. Even elementary textbooks contain information about atomic structure and behavior. By the time most students reach middle school, they are familiar with such terms as "nucleus," "electron," and "radioactivity."
Because we "know" so much about atoms, it is easy to forget that our modern understanding of atoms is built on a century's worth of reasoned inferences made by cautious investigators based on indirect observations. Before going too far in the Atoms and Matter module, then, it is essential:
Hence, the "Whatzit?" activity.
With some advanced preparation, you should be able to make a smooth transition from the Opener to this activity. In addition to the overhead projector you used in the Opener, you will need:
You can make the visual barrier by cutting one side away from a cardboard box large enough to surround the base of the overhead projector as shown in Figure 1.
Making a Visual Barrier to Surround an Overhead Projector
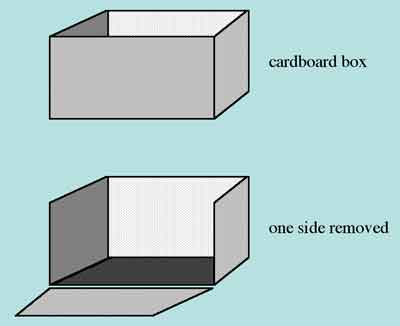
Figure 1. Making a projector shield from a cardboard box.
Alternatively, you can make a three-sided, free-standing screen either by creasing a large sheet of heavy board, or connecting three individual panels with tape.
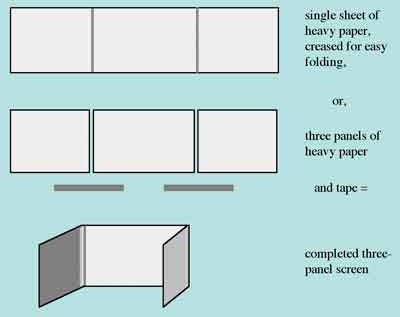
Figure 2. Constructing a free-standing screen out of heavy paper panels.
Have a number of small objects on hand to use as "Whatzits." Here's a list of things that have worked well, but you will probably come up with your own collection of favorites.
Suggested "Whatzits"
A |
B |
C |
D |
scissors |
ball |
Rubik's cube |
pencil |
fork |
film canister |
diskette |
wooden dowel |
leaf |
coin |
pyramid |
letter opener |
flower |
funnel |
milk carton |
paint brush |
protractor |
coffee mug |
square memo pad |
chopstick |
Students may work on this activity individually, but will derive greater benefit from working with a partner. (Two partner pairs can join forces later in the lesson for work on "A Radioactive 'Sandwich'.")
Start out with familiar objects with easily recognizable silhouettes such as those listed in column A. Gradually increase the challenge using objects that may be familiar but which have ambiguous or nondistinctive silhouettes. The objects in column B, for example, will all look round when viewed along one line of sight, but show more readily identifiable silhouettes when turned to reveal their other faces. Similarly, you can place the objects in column C on the overhead so they cast a square shadow on the screen.
You can further increase the challenge by revealing only a small portion of an object at a time. Mask the projector surface with a large piece of opaque paper from which a window (approximately, 10 ´ 10 cm) has been cut. The objects listed in column D are all long and narrow. When only the shadows of their mid-sections are seen, they become virtually indistinguishable from one another. A glimpse at the silhouettes of their "working ends" (brush or pencil point, for example) will make it easier for your students to identify them correctly. And, given an opportunity to re-examine the shadows of these objects' mid-sections, your students will be better equipped to detect more subtle distinguishing characteristics that may have escaped their notice earlier.
d. Activity 2--Radioactive "Sandwich" I
The news of Röntgen's penetrating rays quickly captured the popular imagination. Within weeks, scientists around the world were so completely absorbed in their own X-ray investigations that they nearly overlooked the announcement of an equally surprising discovery by the French physicist, Henri Becquerel (pronounced, ahnh-REE beh-ker-ELL; 1852-1908).
Becquerel was one of the first scientists in the world to see copies of Wilhelm Röntgen's X-ray shadowgraphs. Like his colleagues, Henri was amazed by the ghostly images he saw. But what really intrigued him was the apparent connection Röntgen had found between X-rays and phosphorescence.
NOTE: Some natural substances readily absorb ultraviolet rays, but release that radiation gradually in the form of visible light. Placing these substances in sunlight allows them to soak up the sun's ultraviolet radiation. Later, when brought into a darkened room, they will glow for a time with an eerie light--a greenish-yellow, perhaps, or pink, or blue. This phenomenon is called phosphorescence. Your students will have seen this effect in glow-in-the-dark novelties.
The lingering glow in phosphorescent materials is what distinguishes them from those that fluoresce. Fluorescent materials stop glowing as soon as the external energy source is removed. Students will be familiar with fluorescence in blacklight posters and electric lighting fixtures.
Years earlier, Becquerel's father had investigated the luminous properties of phosphorescent substances. Henri still had the chemicals his father had used. He immediately devised an experimental plan to find out if their phosphorescent glow was in any way related to the production of X-rays.
Background information on Henri Becquerel and his discovery is provided in the reading selection entitled, "Becquerel's Accident," which is intended as a homework reading assignment for your students.
The materials necessary to complete this investigation are very simple, but some items will have to be acquired in advance. In addition to the common items (cardboard and rubber bands) listed in the student activity sheet, each group will need:
Lantern mantles are used to provide bright, incandescent light in kerosene camping lanterns. They look like very small knit white socks with a colorful band at the cuff.
Each group of students will need one mantle to use as their radioactive source. Until recently, lantern mantles commonly contained small amounts of a common isotope of thorium--thorium 232.
NOTE: For more information on thorium-232, see "Isotopes of Thorium" in the Appendix.
In many ways, thorium-232 is an ideal component for lantern mantles: It is abundant, it can withstand lengthy exposure to high temperatures, and it emits a brilliant white light when heated to incandescence. But, like other isotopes of thorium, thorium-232 is naturally radioactive, and some manufacturers now use the non-radioactive element, yttrium, in their lantern mantles. Yttrium-based lantern mantles make terrific camping lantern lights, but they will not work at all in this activity. Thus, before purchasing lantern mantles for this activity, be sure to read the package carefully to make sure it contains thorium.
Sears, Roebuck & Co. still carries thorium-based mantles: Century "Primus," Model 2295, Slip-on (Double-tie) Lantern Mantles. On the back of the package, you should find the following statement: "IMPORTANT: in normal usage, these mantles are quite safe but they contain and emit small amounts of naturally radioactive materials whether lit or unlit...." The thorium compound is concentrated in the colorful cuff of the lantern mantle.
The mantles come 2 to a package (each package retails for under $1.50). Because the half-life of the thorium isotope in lantern mantles is 14 billion years(!), this is one equipment purchase you will only have to make once.
Thorium-232 emits alpha particles, positively charged helium nuclei. Many of the energetic "rays" that Becquerel observed in his experiments were, in fact, alpha particles. Alpha particles are stopped readily by almost any barrier put in their way. They can't even travel far through air before being absorbed. This makes alpha emitters safe to handle as long as proper precautions are taken to avoid extended exposure, or direct contact.
NOTE: ALL alpha emitters MUST be handled carefully to avoid ingestion.
Thorium-232 atoms that find their way into soft human tissue (lungs or digestive tract, for example) will continue to emit alpha particles resulting in damage to the surrounding tissue.
--IMPORTANT SAFETY TIPS-- To avoid ingesting thorium atoms while handling lantern mantles, your students should:
|
To give your students additional incentive to handle the materials appropriately, hold a mantle near a Geiger counter!
NOTE: You may prefer to handle the mantles yourself. Once student groups have all the ingredients for their "radioactive sandwich" ready to assemble, simply walk around the room and, using the same safety precautions described above, distribute one mantle to each group, placing it on one of the cardboard "sandwich" sheets.
You can also perform this activity as a classroom demonstration.
![]() Light-sensitive solargraphic paper
Light-sensitive solargraphic paper
Solargraphic paper is specially treated with light-sensitive chemicals. When you place an object on the paper's surface and then expose it briefly to direct sunlight, a silhouette of the object will soon form. You can stop the chemical action and preserve the shadowy image by simply rinsing the paper thoroughly in water.
The chemicals in solargraphic paper also respond to exposure to alpha particles, although the reaction is quite slow. It may require several hours to produce an identifiable image.
You should be able to find solargraphic paper in your local science museum gift shop, or at a store that specializes in science-oriented items, like The Nature Company, or Natural Wonders.
If you cannot find solargraphic paper locally, you can order some directly from the Lawrence Hall of Science, which produces its own brand, Sunprint®. The folks there are extraordinarily helpful and will answer any questions you may have about their product. Their "Super Sunprint® Kit" retails for roughly $10.00 per package and contains twenty-four 20 x 30 cm sheets of solargraphic paper as well as one 20 x 30 cm acrylic sheet. (Although the acrylic sheet is useful for making silhouettes using sunlight, it is not necessary for this activity.) Each paper sheet can be cut into quarters (10 x 15 cm) providing enough pieces to supply 96 groups of students. Smaller Sunprint ® kits are available. For more information, contact: Lawrence Hall of Science, University of California, Berkeley, California, 94720.
NOTE: Solargraphic paper works best if it is fresh. Paper that has been on the shelf for awhile may produce good images when exposed to sunlight, but may no longer be sensitive enough to respond to alpha particles. Purchase only what you are likely to use in a short period of time.
Be sure to give the activity a dry-run yourself a few weeks before you try it with your students to ensure that they will obtain a good clear shadowgraph.
You should observe a reaction in the solargraphic paper in as little as six hours exposure time. Wait overnight for the best results.
WHAT TO DO... ...if you fail to notice any reaction in your solargraphic paper:
|
Henri Becquerel used photographic plates to record his radioactive shadowgraphs. To make this activity more "authentic," photographic film could be used in place of the solargraphic paper, but it is neither practical nor economical to do so. In the past, it was possible (though messy) to use individual sheets of a self-developing black and white film such as that produced by Polaroid. For purists out there who would like to try the old photographic method, the film can still be obtained. However, it is difficult to find and extremely expensive due largely to popular demand for color film pre-packaged in special camera cartridges.
![]() Small, flat object
Small, flat object
Because alpha particles are so easily absorbed, your students can choose from a wide variety of objects to produce their shadowgraphs. Encourage them to be creative. Many will try a coin, a postage stamp, a paper clip, or a rubber band. These are all objects that will cast a shadow when exposed to a visible light source. But what will happen if the object is transparent to visible light?--a strip of clear, cellophane tape, for example, or a small piece of plastic wrap? What substances are transparent to alpha particles?
![]()
![]()
![]()
Assembling the radioactive sandwich is quite simple and should take only a few minutes. Because the solargraphic paper must be handled somewhat quickly, your students may feel a need to rush. Encourage them to take the time to position the small, flat object carefully on the colored portion of the lantern mantle. A little care taken in this important step will avoid disappointment later. And, remind them to handle the lantern mantle safely!!!
Collect the completed sandwiches and store them in a dark place where they will not be disturbed over night.
e. Closure
Once again, use the X-ray photograph you had shown your students during the lesson's Opener as a visual prompt to guide them in reviewing what they have learned about the value (as well as the limits) of inference in scientific investigation, particularly those investigations involving phenomena that are inaccessible using more direct observational techniques.
Some sample questions are provided in the lesson plan to facilitate discussion. Important points to emphasize during the course of this discussion include:
f. Homework
As part of the "Radioactive 'Sandwich'" activity, your students will have stated what they expect their shadowgraphs to look like based on discussion with other members of their group. In this homework assignment, they will reconsider their earlier prediction after reading the story of "Becquerel's Accident."
Encourage your students to think about the similarities and differences between Becquerel's experiment and what they just did in the "Radioactive 'Sandwich'" activity. After they've had the opportunity to examine the shadowgraph Becquerel made with the uranium salt and the piece of copper, how do they think their own shadowgraph will look?
In addition, a translated excerpt from the paper, "The Invisible Rays Emitted by Phosphorescent Bodies," that Henri Becquerel read before the French Academy of Sciences on March 2, 1896, is included here as supplementary reading. We have adapted the more convoluted phrasing of the original text so that your students can enjoy reading it as well. From it, they will gain insight into the ways that scientists deal with expected and unexpected observations.
Of particular interest is the reproduction of Becquerel's historic photograph showing the silhouette of a cross-shaped copper sheet produced by rays emanating from a piece of uranium salt. Your students can compare their own radioactive shadowgraphs with Becquerel's image.
![]()
3. LESSON PLAN TWO--NEW QUESTIONS a. Concept for the Day
Benchmark issues:
|
b. Opener
Your students will be anxious to open their radioactive "sandwiches." But before they do, invite them to spend a few minutes sharing and discussing the predictions they have made regarding the appearance of their shadowgraphs. Be sure to elicit predictions from those students who used objects that are transparent to visible light.
c. Activity 3--Radioactive "Sandwich" II
Take a few moments to review all safety procedures before distributing the "sandwiches" to your students. The same precautions used in handling the lantern mantles while assembling the "sandwiches" must be employed when students take them apart.
NOTE: Some of your students may be under the mistaken impression that the radioactivity in the lantern mantles has somehow been rendered harmless over night. After all, most natural processes with which they are familiar are of relatively short duration: water evaporates, hot objects cool down, glue dries, cakes bake.... It may seem reasonable to assume that after so many hours spent exposing the solargraphic paper, the lantern mantle will have exhausted its supply of radioactive energy.
It is of utmost importance to be sure your students understand that because of thorium-232's remarkable half-life (14 billion years), and the radioactivity of its decay products, the level of radioactivity each lantern mantle possesses will remain virtually constant.
You can drive this point home by scanning a few of the "sandwiches" with a Geiger counter.
To stop the chemical reaction in the solargraphic paper and "fix" the shadowgraph image, follow the instructions supplied by the paper's manufacturer. Draw your students' attention to the need for care in handling the solargraphic paper while "fixing" the shadowgraphic images. The chemicals which give the solargraphic paper its sensitivity to light can irritate the skin. To protect against this hazard, gloves should be worn throughout the activity.
As soon as your students have disassembled their "sandwiches," collect all lantern mantles in a clearly labeled container (a covered glass jar, or sturdy, sealable plastic storage bag, for example). This can be accomplished simply and efficiently by carrying the container and tongs with you while you circulate among the groups. Take mental notes of each group's results in preparation for the follow-up discussion.
NOTE: Like any other chemical supplies, store the mantles for future use in a cool, dry environment. Be sure to locate them in a low-traffic area away from photographic film or other light-sensitive materials.
Henri Becquerel's discovery is a milestone in the history of science for a number of reasons. The most obvious one, of course, is its role in triggering Marie Curie's pioneering investigations into the atomic structure of matter. However, there is another important lesson to be learned from Becquerel's "accident": Experimental outcomes that do not match expectations can sometimes produce the most interesting discoveries.
Invite groups to share their shadowgraph images with the class. Using Becquerel's example, encourage your students to describe and analyze any differences they found between their shadowgraph expectations and the outcomes they observed.
d. Activity 4--Atoms and Matter Video
The "Atoms and Matter" video links the pioneering discoveries of Wilhelm Röntgen and Henri Becquerel with broader issues related to the role of women in science at the turn of the century.
The drama features a friendly conversation between the young chemist, Marie Curie, and astrophotographer, Margaret Lindsay Huggins. Marie Curie's discoveries in the field of radioactive research have made her name a familiar one. Her perseverance and dedication have inspired generations of young women to pursue a scientific career. By contrast, few people today recognize the name of Margaret Huggins. Despite their differences in age, language, education, and field of study, these two women were alike in many ways. Both shared a love of science and scientific investigation. Both worked collaboratively with their husbands: Marie with the physicist, Pierre Curie, and Margaret with the astrophysicist, William Huggins. And, both were fascinated by the remarkable properties of the new element, radium.
In June 1903, the Curies were invited to London to share what they had learned about radium with their English colleagues. We don't know if Marie and Margaret met during that visit, or even if a conversation of the type that has been created for the video drama actually took place. What we do know is that in 1903, William Huggins was president of the Royal Society of London. As a leader in London's scientific community, he attended social and professional meetings with the Curies during their stay. Happily for Margaret, her role as wife of the Royal Society's president would have obligated her to spend time with Marie Curie. We also know that William and Margaret Huggins began their observations of the spectrum of radium immediately after the Curies' visit to London.
In writing the script for the video drama, we tried to imagine what these two remarkable women might have talked about if they had a few minutes to chat. What is radioactivity? What are the properties of the new element, radium? What is it like to work with your husband?
As your students listen in on this brief encounter, ask them to look for similarities and differences between these two women. What do Marie Curie and Margaret Huggins want to know more about?
f. Closure
Where do scientists' questions about the natural world come from? Wilhelm Röntgen and Henri Becquerel were the first to take notice of previously unimagined phenomena. When they got over the sense of surprise and disorientation that accompanied these discoveries, how did they organize their thoughts? How did they describe what they had seen to others when there really were no words that quite fit the situation? How did a plan to investigate these phenomena further take shape in their minds?
Befuddlement is an important part of the scientific inquiry. It is a stage filled with confusion, excitement, missteps, frustration, and intellectual challenge. All too often, after things settle down in the investigative process, scientists tend to "forget" about the initial struggles they faced. The story of a discovery becomes a sequence of smooth transitions from observation to description to investigation and finally to explanation. The initial uncertainties and insecurities fade into invisibility.
Use this closure discussion to focus your students' attention on the questions asked and answered in the video dialogue about the remarkable behavior of the new element, radium. Some sample prompts are provided in the lesson plan to help you get the discussion started.
Be sure to have your students reflect on the questions that arose in their own minds after seeing the image that formed on their solargraphic paper in the "Radioactive 'Sandwich'" activity. Encourage them to focus on the task of translating their casual observations into fruitful questions.
g. Homework
A variety of news items provided in the Student Reader for your students' reference to give them a sense of what it was like to live in 1903. Included are:
This map should help students understand more about the world that Marie Curie and Margaret Huggins lived in. Notice the locations of the cities London, Paris, and Warsaw. Notice the boundaries of Poland, Marie Curie's homeland.
How do the boundaries of Poland shown on a modern map of Europe compare to its boundaries in 1903? How far is it from Warsaw to Paris? How far is it from Paris to London? How do you think people traveled long distances in 1903?
The basic structure of the periodic table familiar to chemists in 1903 seems very similar to the one we use today. What looks the same? What is different? Why were these changes made? Who made them? When? What is the element "niton"? "Columbium"? Why are some elements found on the modern periodic table, like francium, plutonium, and promethium missing from the 1903 table?
A partial list of news-worthy events that occurred in the United States between 1900 and 1905. Excerpts from news articles about some of the events on that list are described below, along with suggested guide questions.
Ever wonder why cars have license plates? Students may find it interesting to read about a time when there were so few automobiles. Even back in 1903, drivers could be rude!
What problems do modern drivers face while on the road? What regulations have local communities adopted to control such things as speed, highway conditions, driver qualifications, vehicle maintenance?
If the events described in this news article occurred on May 31, 2003, what facts in the story would likely be different? What would likely stay the same? How would flood victims be rescued? How would communications and transportation be affected? How would the descriptive language used in the article change?
How does this advertisement for an automobile compare to those in today's newspapers? Buying a new car for only $2500 seems like quite a bargain today, but what would this price be equivalent to in today's dollars?
It took two months and one day, but E. T. Fetch completed the second, but fastest, transcontinental trip made by a gasoline powered automobile on August 21, 1903.
How did the road conditions back then compare with today's interstate highway system? How many miles is it from San Francisco to New York? How long would a similar trip take today?
How does this article compare with a similar story about a modern feat of daring, or technological first?
The report in the New York Times on the outcome of baseball's first World Championship series ran a total of 4.75 column inches! We suspect the report in the Boston papers was a bit more thorough as they announced their team's victory over Pittsburgh.
How does this article compare with a report about a modern championship game in any sport.
The fire which destroyed Chicago's new Iroquois Theatre took the lives of 588 people, many of them women and children who were enjoying a holiday matinee. To prevent future disasters, new safety codes were enacted in many cities requiring theaters to have more fire walls, better and more exits, unobstructed alleyways, and non-flammable scenery.
Why are fire drills so important in schools? Why is walking out of a burning building safer than running? When the lights go down in a theater, why do the exit signs always stay lit up?
Here's an article about an inventor and his claim to have invented the "telephotograph," a telephone that transmits images as well as sounds. If you could interview this inventor, what questions would you ask? Based on the information in the article, how do you think his "telephotograph" really works? When was the first successful demonstration of an image carrying telephone?
The first underground passenger railway opened in London in 1863. The success of this means for transporting people quickly and safely across a city whose surface streets were hopelessly clogged by traffic of all description inspired officials in America's large cities to undertake a similar venture. In 1897, Boston became the first, carrying passengers on a 1-1/2 mile underground rail line. It took another seven years before the first line of New York's subway system became operational.
What other cities have built subway systems? How big is New York City's subway system today? How many passengers do subways carry each day? What new methods of rapid transit are being tried today?
There are a number of interesting writing challenges that you can assign your students based on these readings. Here are a few suggestions:
![]()
4. LESSON PLAN THREE--WOMEN IN SCIENCE a. Concept for the Day
Benchmark issues:
|
b. Opener
Encourage your students to share what they learned from their reading about life in 1903. Use this opportunity to help them make good comparisons between life today and life back then. From the information on details contained in each document, students should be able to identify both similarities and differences between past and present. From these details, they should be able to both state some generalizations about life then and now, and formulate some additional questions for further investigation.
c. Activity 5--Women In Science, Part I
The Student Reader contains a total of 65 brief informative reading selections related to the lives and work of Margaret Huggins and Marie Curie from which your students may choose for this activity. The readings are organized topically within four major categories: Science and Education for Girls and Women in the Nineteenth Century; Margaret Huggins; Marie Curie; and Radium.
(1.) Science and Education for Girls and Women in the Nineteenth Century
(a.) Excerpts from:
- "Women's Friendships", Saturday Review (1864);
- Sesame and Lilies, by John Ruskin (1865);
- The Higher Education of Women, by Emilie Davies (1866);
- "Miss Becker on the Mental Characteristics of the Sexes," The Lancet(1868); and
- "To Educate Young Women Like Young Men," by John W. Burgon (1884).
(b.) Biographical essays on:
- Angeline Hall (1830-1892); and
- Isobel Gill (ca. 1870).
(2.) Margaret Huggins
(a.) Biographical essay on Margaret Lindsay Huggins (1848-1915)
Divided into eight brief sections. Accompanied by five illustrations including a photograph of Margaret Huggins and reproductions of her first entries in the observatory notebook.
(b.) Excerpts from Good Words:
- "On Light," by Sir John F. W. Herschel, May 1, 1865; and
- "A True Story of the Atmosphere of a World on Fire," by The Reverend Charles Pritchard, April 1, 1867.
(c.) Biographical essay on William Huggins (1824-1910)
Divided into seven brief sections. Accompanied by four illustrations including a photograph of William Huggins and a reproduction of the title page of his first observatory notebook.
(d.) William Huggins' Correspondence on the Subject of Radium
Ten brief letters on the subject of radium from William Huggins, with occasional remarks by Margaret Huggins, to such notable scientists as Joseph Larmor, Arthur Schuster, and George Ellery Hale.
(e.) A letter from Marie Curie to Margaret Huggins, December 6, 1903.
In French, followed by an English translation. Marie Curie thanks the Hugginses for their hospitality to Pierre on a visit he had recently made to London.
(3.) Marie Curie
(a.) Biographical essay on Marie Curie (1867-1834):
- "Manya"
Divided into four brief sections dealing with Marie Curie's life between 1867 and 1883. Accompanied by two illustrations: a photograph of Marie Curie and a map of Russian Poland.
- "Mademoiselle Marie Sklodowska"
Divided into three brief sections dealing with Marie Curie's life between 1883 and 1895. Accompanied by a map showing the geographical relationship of Warsaw to Paris.
- "Madame Marie Curie"
Divided into six brief sections dealing with Marie Curie's life between 1895 and 1903. Accompanied by two photographs of Marie and Pierre Curie.
(b.) Biographical essay on Pierre Curie (1859-1906):
Divided into five brief sections. Accompanied by a photograph of Marie and her daughters after Pierre's death.
(c.) News articles on the death of Pierre Curie:
- "Prof. Curie Killed in a Paris Street," from the New York Times, April 20, 1906; and
- "Pension for Curie Family," from the New York Times, April 22, 1906.
(4.) Radium
(a.) "The Effects of Radium":
- Report by Pierre Curie on effects of exposing his arm to radium; and
- Report by Marie Curie on the problem of radioactive contamination.
(b.) News articles on the subject of radium:
- "The Mystery of Radium," London Times, March 30, 1903;
- "Professor Curie on Radium," London Times, June 20, 1903;
- "Royal Society's Conversazione," London Times, June 20, 1903;
- "Successful Application of Radium," London Times, July 4, 1903;
- "Radium and Helium," London Times, July 20, 1903;
- "The Mystery of Radium," London Times, August 13, 1903;
- "To Make Luminous Drinks from Radium," New York Times, January 14, 1904; and
- "'Liquid Sunshine' on Tap," New York Times, February 5, 1904.
During this investigative phase of the "Women in Science" activity, give your students a few minutes to examine the readings available in the Student Reader and make their selections. The item a student selects will automatically place him or her in a report group.
Circulate among the groups as they do their research and begin assembling their group reports. It may be difficult for students to agree on the three most important pieces of information that they have gathered from their reading. Encourage them to think about ways of resolving their differences; of prioritizing their individual choices and reaching consensus; of sharing responsibility for creating the report.
d. Closure
No matter how well your students absorb and internalize new information, it is always a challenge for them to put that information into perspective--to see the big picture when the details seem so concrete and learnable. All the information your students gathered in today's activity will become a collection of mere trivia and factoids to be ejected from their mental file drawers ten minutes after the unit test if the larger issues at stake remain undiscovered and unappreciated.
Use the discussion in the closure to focus your students' thoughts on the principal concept of today's lesson, namely, that despite obstacles, women pursued their scientific interests and made valuable contributions. In particular, the examples of Marie Curie and Margaret Huggins show that women have created opportunities for themselves to pursue their personal interests in scientific investigation.
e. Homework
In today's activity, your students had a glimpse of daily life in another time. The time in question was a century ago. It is difficult for young people to really feel at home in such a distant historical situation.
For homework, ask your students to interview an older person they know about what life was like when that person was their age. Suggested interview questions are provided in Lesson Plan Three.
The purpose of this homework assignment is to help your students recognize that they have direct access to information about life in another time through older people they know. Using these contacts as bridges to more recent historic events, your students will develop their ability to build meaningful connections to the more distant past.
![]()
5. LESSON PLAN FOUR--SOLVING PUZZLES a. Concept for the Day
Benchmark issues:
|
b. Opener
The discovery of radioactivity marked the beginning of what some historians have called the Age of Bewilderment for physicists and chemists alike. It is not so easy for adults who have grown up in the atomic age to fully appreciate the awkward mix of powerlessness and excitement felt by the Curies as they stood on the edge of terra incognita. But so much about the world is bewildering to young people that your students will see themselves standing alongside these pioneers.
What lies beyond the edge? One way to find out is to step past it. If that's not possible, clues about what's over there have to be gathered indirectly.
The opening discussion focuses on jigsaw puzzles, an amusement with which most of your students will be familiar. If you want to make the discussion a bit more concrete, use a real jigsaw puzzle as a visual prompt and referent. Dump all the pieces out on a table or other surface. Pick through the pieces the way you would if you were beginning to work on it. As you do, your students will guide you on what to do next. Introduce examples of problematic pieces which commonly confront puzzle solvers--pieces from an area in the puzzle that is all one color, pieces that don't seem to fit anywhere, pieces that look like they belong to another puzzle....
Use this opener to pull your students in to the joy, the challenge, the frustration, and the excitement of puzzle solving. Help them recognize that science research is like working on a gigantic jigsaw puzzle, but a puzzle with no picture on its box to go by, no edge pieces, or clue as to how many pieces there are.
c. Activity 6--Women In Science, Part II
The "Whatzit" and "Radioactive 'Sandwich'" activities exposed your students to examples of scientific puzzles that rely heavily on inference from indirect evidence for their solutions. As your students share what they have learned from the historical documents they examined, be sure to draw their attention to the fact that these documents are another type of indirect evidence. Remind them that their reports are based on inference from this evidence. Encourage them to think critically about the reliability of the information in the documents they have read. Help them distinguish between fact and opinion.
Moderate the delivery of reports. Each group should only take 3-5 minutes. Follow up each group report with a brief discussion period. Use these suggested questions as guidelines for framing your own:
(1.) Science and Education for Girls and Women in the Nineteenth Century
(a.) The proper Victorian lady was undoubtedly as much a social fiction of the late-nineteenth century as June Cleaver and Harriet Nelson are of the mid-twentieth. In the writing of these social commentators, we catch a glimpse of how people in the nineteenth century believed that fiction could be realized through education.
- What is the ideal woman in today's society?
- How do today's ideals compare to those of the nineteenth century?
- How do you think girls should be educated?
- Do you think girls can be scientists?
(b.) Other women in Margaret Huggins' generation worked collaboratively with their astronomer husbands. Angeline Hall was the wife of Asaph Hall, an American astronomer who is credited with having discovered the moons of Mars. As this essay shows, Angeline's education and support was of great benefit to her husband in his research. Isobel Gill traveled with her husband, David Gill, on a number of astronomical expeditions. For many years, the Gills lived in South Africa while David directed the Capetown Observatory. Isobel's story shows the pluck and courage that was sometimes required of wives on such expeditions.
- What did Angeline Hall and Isobel Gill contribute to their husbands' astronomical research?
- What other jobs require collaboration?
- What is an "opposition" of Mars?
- Why are astronomers so interested in observing Mars during an opposition?
(2.) Margaret Huggins
(a.) Margaret Lindsay Huggins
- Where and when was Margaret born?
- What was required to take a photograph when Margaret was young?
- How do you think Margaret became interested in astronomy? What evidence did you use to draw your conclusion?
- What discoveries was William Huggins known for?
- Where was the Hugginses' observatory located?
- What did Margaret contribute to the work of the observatory?
- Why did Margaret want to take a photograph of a nebula's spectrum?
- Why is it likely that Margaret Huggins met Marie Curie in June 1903?
(b.) Good Words
Aside from George Airy, the Astronomer Royal, John Herschel was probably the best known and most highly respected English astronomer alive in 1865. He came from a family of astronomers. His father, William Herschel was a master musician and telescope builder who became famous after he discovered the planet Uranus in 1781. His aunt, Caroline Herschel, was an astronomer, too. She discovered eight comets.
- John Herschel provides instructions for making a special observing instrument. What does this instrument do? What would we call it today?
- How easy do you think it would be to follow Herschel's instructions?
In 1867, Charles Pritchard was the president of the Royal Astronomical Society. He was not as well-known as John Herschel, but he was a highly respected amateur astronomer.
- What is the title of this essay? What effect did reading the title have on you?
- What is the "world on fire" that Charles Pritchard is referring to? What would we call such an event today?
- Why is Charles Pritchard so excited about science?
- Pritchard talks about astronomy being pushed in a new direction. How has astronomy changed in recent years? Why?
- What experiment does Pritchard describe?
- How easy do you think it would be to follow his directions?
(c.) William Huggins
- Where and when was William Huggins born?
- What kind of education did William Huggins receive?
- How do you think William Huggins became interested in astronomy? What evidence did you use to draw your conclusion?
- What is an "amateur"?
- Do you think William Huggins liked astronomy? What evidence did you use to draw your conclusion?
- What did William Huggins' observatory have in common with a chemical laboratory?
- What new questions could astronomers ask because of William Huggins' pioneering work?
- What is the name of the instrument that William Huggins attached to his telescope?
- Who helped him in his early study of the chemistry of stars?
- Who helped him in his astronomical work after 1875?
- What official position did William Huggins hold in 1903?
- What is a "conversazione"?
(d.) Correspondence on the Subject of Radium
- Who was Joseph Larmor?
- What does William Huggins ask Joseph Larmor at the end of the letter dated July 16, 1903?
- In his letter dated July 19, 1903, why does William Huggins say he is having a problem comparing the spectrum of radium glow with that of helium?
- What questions does he have about electrons? About radium?
- Who was William Ramsay?
- What does William Ramsay think that radium might be? (See letter dated July 20, 1903.)
- What trouble does William Huggins report in his letter dated July 24, 1903?
- Who was Ernest Rutherford?
- Do you think that William Huggins is discouraged by the problems he describes?
- Who was Carl Runge?
- Who was George Ellery Hale?
- What does Hale tell William Huggins in his letter dated August 10, 1903?
- How did scientists get information about the research that other scientists were doing?
- Who is Arthur Schuster?
- How does William Huggins describe the difference between the German and French samples of radium bromide?
- How does William Huggins describe the appearance of the surface of a radium sample in his letter dated October 28, 1903?
- What is the subject of the letter dated May 2, 1906?
- Why was William Huggins upset about the cost of radium?
- How does William Huggins describe Marie Curie in his letter dated August 17, 1908?
(e.) Letter from Marie Curie to Margaret Huggins,
- Why did Marie Curie write a letter to Margaret Huggins?
- How do you think Marie Curie feels about husbands and wives working together on scientific research projects? What evidence did you use to support your conclusion?
- What special gift did Marie Curie send to Margaret Huggins?
(3.) Marie Curie
(a.) Marie Sklodowska Curie
- Where and when was Marie Sklodowska born?
- Why has Polish history been so turbulent?
- How do you think Marie became interested in science? What evidence did you use to support your conclusion?
- How did Marie's mother die?
- Why was it unfortunate that Marie was interested in continuing her studies after high school?
- How did Marie and her sister, Bronya, continue their educations?
- Why did Marie have to carry a blanket and a stool on her train trip from Warsaw to Paris?
- How did student life in Paris compare to that in Warsaw?
- How did Marie save on her living costs when she was a student?
- What research work did she undertake after receiving her physics diploma?
- What problem did she face when she started her research?
- How did she solve her problem?
- Why did Pierre Curie want Marie to return to Paris?
- How did Pierre and Marie Curie travel around France during their honeymoon?
- What did Marie have to do before she could earn her doctorate?
- What did Marie choose to study for her original research project?
- Why was it risky to study something so new?
- What new questions arose from her study of radioactivity?
- What is pitchblende?
- Why was Marie surprised by the results of her investigation of pitchblende?
- What did Marie name the first new element she discovered? Why?
- What did Marie name the second new element she discovered? Why?
- Why did Pierre Curie give the lecture on radium at the Royal Institution even though Marie did most of the research?
- What did Pierre Curie show his English colleagues during his lecture?
(b.) Pierre Curie
- Where and when was Pierre Curie born?
- How do you think Pierre Curie became interested in science? What evidence did you use to draw your conclusions?
- Why did Pierre like science so much?
- What important discovery did Pierre make?
- What was Pierre's reaction to the news that he had been nominated for the Nobel Prize?
- Who was awarded the Nobel Prize for physics in 1903? Why?
- What happened in April 1906 that changed the life of Marie Curie?
(c.) News articles on the death of Pierre Curie
- How was Pierre Curie killed?
- Why were the Curies trying to find less expensive ways to produce radium?
- Why did Pierre Curie frequently have to delay his research?
- Why did Pierre Curie refuse the award of the French Legion of Honor for his important scientific work?
- How did the French government provide for Marie Curie and her two daughters after Pierre's death?
(4.) Radium
(a.) "The Effects of Radium"
- What happened to the skin on Pierre Curie's arm after he exposed it to radium?
- How long did it take for the wound to form a scab?
- How did the wound appear nearly two months after exposure to radium?
- How did Pierre Curie describe the experience of Marie Curie after being exposed to radium for only a half an hour?
- How does Pierre Curie describe the effect of handling radium on his hands?
- What problem did Marie Curie encounter in her attempts to continue her study of radium?
(b.) News articles on the subject of radium
- Why does Johnstone Stoney describe radium as a mystery in his letter to the London Times published March 30, 1903?
- How does the London Times (June 20, 1903) describe Pierre Curie's talk at the Royal Institution?
- How did Pierre Curie demonstrate that radium emits heat?
- What medical use was found for radium? (See London Times, July 4, 1903.)
- With what instrument did William and Margaret Huggins study radium? (See London Times, July 20, 1903.)
- What evidence did the Hugginses find to link helium with radium?
- Who else found a connection between helium and radium in their research?
- What did the scientific community understand about radium in 1903?
- How much did the general public know about the properties of radium at that time?
- What new uses for radium were predicted by scientists in the United States? (See New York Times, January 14, 1904.)
- How did scientists amuse themselves with irradiated liquid? (See New York Times, February 5, 1904.)
- Do you think people understood the risks of exposure to radium at that time? What evidence did you use to draw your conclusions?
d. Closure
A lot of new and unfamiliar information will have entered your students' ears during today's lesson. The goal in the closing discussion is to help that information form a permanent and positive impression in their minds as well.
It is essential to focus your students' attention on the common threads that bind together the seemingly disparate bits of information they have encountered in Lessons Three and Four. One good way to accomplish that is to start an argument, or make a statement that is sure to generate a wide range of opinion. Use the following statements as starting points for discussion:
e. Homework
The next lesson will introduce your students to the phenomenon of radioactive decay and the concept of half-life as a measure of the rate at which a radioactive element releases its energy. Radioactive decay is a probabilistic phenomenon. Although the decay of an individual atom cannot be predicted in advance, the study of large collections of such atoms has made it possible to state the probability that an individual atom will decay within a certain amount of time.
The "'Heads' or 'Tails'?" activity will give your students a little personal experience with the basic challenges of describing and predicting events governed by the laws of chance.
![]()
6. LESSON PLAN FIVE--RADIOACTIVE DECAY a. Concept for the Day
Benchmark issues:
|
b. Opener
The purpose of this opening exercise is to bolster your students' understanding that the outcomes of individual chance events cannot be predicted with absolute certainty. Collecting data on large populations of such chance events makes it possible to determine the probability of an event's occurrence.
Each of your students will have recorded data from twenty coin tosses and tallied their outcomes in the "COIN TOSS TALLY" table. Be sure to draw their attention to the range of variation in the "heads" to "tails" ratios in columns A-E (each representing 4 tosses). Have them compare these ratios with that of their total "heads" and "tails" outcomes (results from 20 tosses).
To give your students the opportunity to enlarge their individual data samples, organize them into groups (preferably five students per group). After summing their individual results, the group should find that the combined "heads" to "tails" ratio is very close to one (results from 100 tosses).
Have groups report their combined totals and sum these for the class as a whole. Calculate the "heads" to "tails" ratio for this large sample.
To further drive the point home, take out a penny and ask your students to estimate their chance of correctly predicting the outcome of a single coin toss.
Now, ask them to estimate their chance of correctly predicting the next class's "heads" to "tails" ratio.
c. Activity 7--____________ 's Half-life
Around the time that Marie Curie was isolating radium, other scientists were investigating radioactivity's remarkable properties. The English scientist, Ernest Rutherford (1871-1937) suspected that an element's radioactivity caused, or was caused by, a major change in the element itself. His study of the radioactive element thorium confirmed his suspicions.
When an atom of thorium-232 emits an alpha particle (helium nucleus = 2 protons + 2 neutrons), for example, it becomes an atom of an entirely different element--radium-228! Eventually, all the thorium-232 in a sample will undergo this transformation. Rutherford called this process of change "radioactive decay."
Radium-228 is also radioactive. When it decays, it emits a beta particle (high energy electron) and becomes actinium-228, another radioactive element. The decay process continues until an atom of a stable element (in this case, lead-208) is produced.
Each decay event is a completely random occurrence. There is no way to predict exactly when one particular atom will decay. Even so, the number of atoms in a measurable quantity of a substance is so large that it is possible to determine how long it will take for, say, half of them to decay.
NOTE: How numerous are atoms? For any given sample of an element, the number of atoms it contains can be easily calculated. Take radium, for example. Its atomic weight is 226.05, which simply means that it is 226.05 times heavier than an atom of hydrogen.
If, by some miraculous process, you were able to accumulate 226.05 grams of radium, you would have one gram-atomic weight of radium. Each gram-atomic weight of an element has the same number of atoms in it. That number is called Avogadro's number in honor of the Italian physicist, Amadeo Avogadro (1776-1856) whose ideas on the structure of matter led to this important principle. Modern methods have made it possible to determine the value of Avogadro's number:
Avogadro's number = 6.02 x 1023.
If 226.05 grams of radium contains 6.02 x 1023 atoms, how many atoms were in the one-tenth gram of radium that Marie Curie isolated?
6.02 x 1023 atoms
|
x atoms
|
|
————————— |
= | ——————— |
226.05 grams of radium |
0.1grams of radium |
x = 2.7 x 1020 atoms in one-tenth gram of radium.
On average, nearly 4 billion of these atoms decay every second. By contrast, only about 1200 atoms in one-tenth gram of uranium decay every second.
Each radioactive element decays at its own rate. This rate is an important property of each element. Scientists have adopted a useful convention to quantify this rate, namely, the amount of time it takes for one half of a pure sample of a particular radioactive element to decay. This time is called that element's half-life.
HALF-LIFE TABLE
FOR LONG-LIVED ISOTOPES OF ELEMENTS 84-112.
Atomic Number |
Symbol |
Longest-lived isotope |
Half-life |
84 |
Po |
Polonium-209 |
103 yrs |
85 |
At |
Astatine-210 |
8.3 hrs |
86 |
Rn |
Radon-222 |
3.8 dys |
87 |
Fr |
Francium-223 |
22 mins |
88 |
Ra |
Radium-226 |
1,620 yrs |
89 |
Ac |
Actinium-227 |
21.2 yrs |
90 |
Th |
Thorium-232 |
14,000,000,000 yrs |
91 |
Pa |
Protactinium-231 |
32,480 yrs |
92 |
U |
Uranium-235 |
713,000,000 yrs |
92 |
U |
Uranium-238 |
4,500,000,000 yrs |
93 |
Np |
Neptunium-237 |
2,140,000 yrs |
94 |
Pu |
Plutonium-242 |
37,900 yrs |
95 |
Am |
Americium-243 |
7,650 yrs |
96 |
Cm |
Curium-247 |
40,000,000 yrs |
97 |
Bk |
Berkelium-247 |
10,000 yrs |
98 |
Cf |
Californium-251 |
800 yrs |
99 |
Es |
Einsteinium-254 |
480 dys |
100 |
Fm |
Fermium-253 |
4.5 dys |
101 |
Md |
Mendelevium-256 |
1.5 hrs |
102 |
No |
Nobelium-253 |
10 mins |
103 |
Lr |
Lawrencium-257 |
8 secs |
104 |
Rf |
Rutherfordium-261 |
65 secs |
105 |
Db |
Dubnium-262 |
34 secs |
106 |
Sg |
Seaborgium-266 |
20 secs |
107 |
Bh |
Bohrium-264 |
.44 secs |
108 |
Hs |
Hassium-269 |
9.3 secs |
109 |
Mt |
Meitnerium-268 |
.07 secs |
110 |
_______-271 |
.06 secs |
|
111 |
_______-272 |
1.5 msecs |
|
112 |
_______-277 |
.24 msecs |
There are many misconceptions related to the concept of half-life. Perhaps the most prevalent is that after two of an element's half-life intervals have elapsed, all the original atoms will have decayed. This erroneous understanding of the meaning of radioactive half-life is depicted in Figure 3.
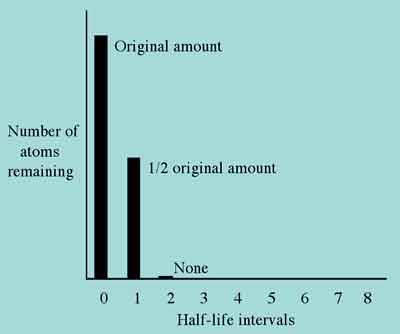
Figure 3. Incorrect understanding of radioactive half-life.
The "________'s Half-life" activity will go a long way toward disabusing your students of this idea. Figure 4 correctly depicts the rate of decay of a radioactive element. From this chart, it is clear that after two half-life intervals, one-quarter of the original number of radioactive atoms will remain undecayed.
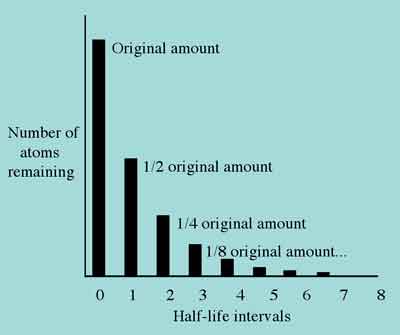
Figure 4. Correct understanding of radioactive half-life.
Introduce the "________'s Half-life" activity by showing a brief clip from the "Atoms and Matter" video in which Marie Curie describes what little she knows about the properties of the rays that emanate from radium. Alternatively, you, or two of your students, can read aloud from the relevant segment of the script ( excerpt is provided in the lesson plan).
Ideally, students should work in pairs on this activity. Achieving this ideal will probably require planning and some adept classroom management on your part. If the number of pennies you have available is limited, you can divide them up into smaller quantities ( preferably no fewer than 50 pennies per group). The results will be the same, although the students may not have quite as much fun shaking their boxes. Because the time required for each group to complete its data collection is quite small, several different groups can take turns using one particular set of pennies.
NOTE: This activity is very noisy if pennies are used. Quieter, two-sided objects can be used if excess noise will get you in trouble with your neighboring teachers. But, please be assured that the noise is something that students like about this activity--and it only lasts a short time.
The following table and graph show the results we obtained starting with 200 "heads-up" pennies to represent atoms of the element "Ahzbium":
HALF-LIFE DATA TABLE: AHZBIUM
Time |
Number of Atoms (Pennies) |
||
Actual
|
Represented
|
Decayed
|
Remaining
|
0 |
0 |
0 |
200 |
10 |
10,000 |
102 |
98 |
20 |
20,000 |
55 |
43 |
30 |
30,000 |
25 |
18 |
40 |
40,000 |
6 |
12 |
50 |
50,000 |
8 |
4 |
60 |
60,000 |
3 |
1 |
70 |
70,000 |
0 |
1 |
80 |
80,000 |
0 |
1 |
90 |
90,000 |
1 |
0 |
100 |
100,000 |
||
GRAPH OF HALF-LIFE DATA: AHZBIUM
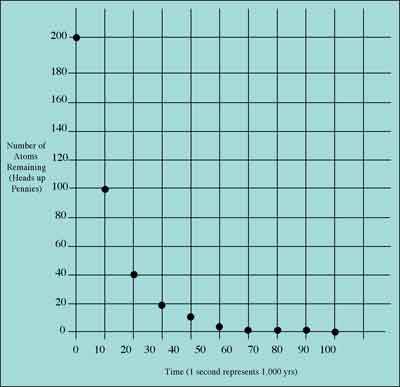
After examining these data, you can estimate Ahzbium's half-life to be about 10,000 years. Does an element's half-life depend on how many atoms you have to start with? (What if you had started with only 100 pennies? 50 pennies?)
d. Closure
If the object of this lesson is to teach your students about radioactive decay, then why bother with pennies? Why not study radioactive materials directly?
The flip of a coin is an inexpensive, safe, fast, and easy way to simulate a random event like radioactive decay. Scientists frequently rely on models of natural phenomena as investigative resources from which they can draw inferences about the particular phenomenon under study.
So far, your students have only dealt with random events involving two possible outcomes. A die has six sides, and thus six possible outcomes from each roll. You may wish to conclude the closing discussion by sparking your students' curiosity about the effect of introducing more possible outcomes into activities like "'Heads' or 'Tails'?" and "_________'s Half-life."
How would they begin to investigate this new situation? What equipment would they need? What procedures would they follow? What results do they expect to find?
NOTE: Don't provide too much information at this time. The main purpose here is to present your students with a puzzle that will get their creative juices flowing. The opener for Lesson Six contains some suggestions on how you can follow-up on this teaser.
e. Homework
Students will plot the data their group collected during the "_________'s Half-life" activity on the graph provided in the activity worksheet. This task will give them practice in converting raw numerical data into a graphic display. Pictorial representation of these data renders the pattern of change in radioactive decay readily visible, and thus facilitates interpretation and understanding.
![]()
7. LESSON PLAN SIX--COLLABORATION a. Concept for the Day Collaboration is often required for successful investigation of natural phenomena. Benchmark issues:
|
b. Opener
In Lesson Five's closing discussion, you asked students to ponder the effect of substituting dice for coins in the "_________'s Half-life" activity. In today's opening activity you will give your students an opportunity to check their own thinking on this interesting puzzle and to further examine the results they obtained in the previous lesson's activity.
Provide each of your students with a die, or a small cube (a wooden block, for example) that has a distinguishing mark on one of its faces. Each of these objects will represent a radioactive atom.
If using dice for this exercise, select one face (a roll of three, for example) that will represent the fact that the atom has experienced radioactive decay. If using a block, the appearance of its marked face will signal a decay event.
Have the whole class roll their dice, or marked blocks. Those whose die or block indicates decay has taken place will sit out the subsequent rounds.
Ask for a show of hands of all others and record their number.
Repeat this until all "atoms" have "decayed."
By quickly plotting the numbers that have been recorded on a simple graph, you can demonstrate to your students that the basic shape of the curve is identical to the one they plotted in the "_________'s Half-life" activity. The main difference will be in terms of the rate at which the decay process is completed.
The following table and graph show the results we obtained starting with 72 dice to represent atoms of the element "Wombattium":
HALF-LIFE DATA TABLE: WOMBATTIUM
Time |
Number of Atoms (Dice) |
||
(in seconds) |
(in years) |
Decayed |
Remaining |
0 |
0 |
0 |
72 |
10 |
10,000 |
9 |
63 |
20 |
20,000 |
14 |
49 |
30 |
30,000 |
11 |
38 |
40 |
40,000 |
2 |
36 |
50 |
50,000 |
7 |
29 |
60 |
60,000 |
3 |
26 |
70 |
70,000 |
5 |
21 |
80 |
80,000 |
4 |
17 |
90 |
90,000 |
2 |
15 |
100 |
100,000 |
4 |
11 |
110 |
110,000 |
3 |
8 |
120 |
120,000 |
3 |
5 |
130 |
130,000 |
1 |
4 |
140 |
140,000 |
2 |
2 |
150 |
150,000 |
0 |
2 |
160 |
160,000 |
0 |
2 |
170 |
170,000 |
0 |
2 |
180 |
180,000 |
0 |
2 |
190 |
190,000 |
0 |
2 |
200 |
200,000 |
1 |
1 |
210 |
210,000 |
0 |
1 |
220 |
220,000 |
0 |
1 |
230 |
230,000 |
0 |
1 |
240 |
240,000 |
0 |
1 |
250 |
250,000 |
0 |
1 |
260 |
260,000 |
0 |
1 |
270 |
270,000 |
1 |
0 |
280 |
280,000 |
||
GRAPH OF HALF-LIFE DATA: WOMBATTIUM
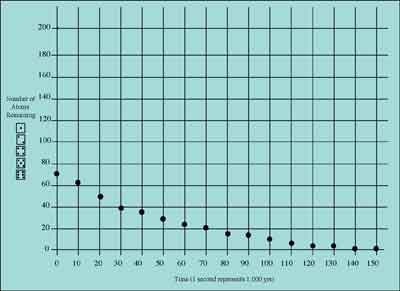
After examining these data, you can estimate Wombattium's half-life to be about 40,000 years.
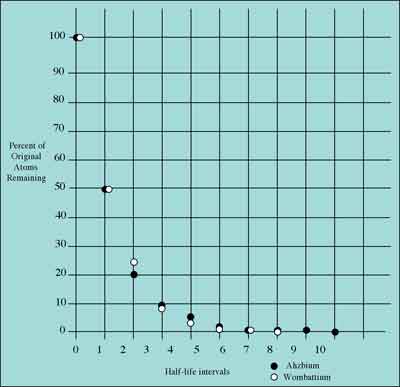
Notice the basic similarity in shape between the half-life graphs for Ahzbium and Wombattium. Both decrease geometrically. But Ahzbium's half-life graph is steep while Wombattium's shows a more gradual decline. To make a more direct comparison between these two graphs, simply:
The following graph shows such a comparison between Ahzbium and Wombattium. The pattern depicted here will be the same for all radioactive elements regardless of their rate of decay or the number of atoms they contain.
THE PATTERN OF RADIOACTIVE HALF-LIFE
Some further questions for you and your students to consider:
c. Activity 8--Two Hands Are Better Than One
One of the important themes in the "Atoms and Matter" module is that scientific investigation often requires teamwork. Learning to rely on others and the skills they bring to a research effort takes time, trust, and lots of practice. "Two Hands Are Better Than One," is a quick and simple activity involving a task your students know they can accomplish all by themselves under normal circumstances--tying a bow in a piece of string in under three minutes.
But, as your students will soon discover, this is not a normal situation. In "Two Hands Are Better Than One" they will be required to complete this task using just one hand and their ingenuity.
A few of your students will succeed in tying a bow single-handedly, but it will be a struggle. Others may realize that they can more easily accomplish the task if they pair up with someone else--preferably someone with complementary ability, that is, using an opposing hand.
This activity should only take a very few minutes--students will grasp the point quickly whether or not they succeed in tying the string.
d. Activity 9--Artists and Talkers
After the "Two Hands Are Better Than One" activity, your students should be in the right frame of mind for the major activity of the lesson: working in pairs to observe and record the spectra produced by a light source when viewed through a diffraction grating.
![]() Light source:
Light source:
Single-filament lamps (aquarium lamps) are excellent light sources for this activity. They are commonly available, inexpensive, require no special equipment to operate, and emit a clear white line of light. For variety, your students can view these white light sources through different colored filters.
NOTE: Avoid using frosted bulbs of any kind as the light produced is too diffuse.
Plücker tubes (pronounced p'LOOK-er; named after the German physicist, Julius Plücker) are long, thin, glass tubes used to study the spectra of light produced by different elements. Each tube is filled with a gas such as hydrogen, helium, mercury vapor, or neon. The gas inside can be identified by observing the distinctive spectrum it produces when high voltage is applied to the tube.
Plücker tubes are excellent alternative light sources to use for this activity, providing you have some on hand, and the high voltage power source to operate them. However, because Plücker tubes are expensive and very fragile, you may wish to introduce your students to them in a classroom demonstration. Simply insert a Plücker tube in the power source, darken your classroom lights, and turn on the power source. From their seats, wearing their rainbow glasses, your students will be able to see firsthand the distinguishing spectral patterns that different elements produce.
Start with a tube filled with hydrogen. To the naked eye, hydrogen gas glows with a pinkish-purple light. Your students may expect to see a pink and purple rainbow when they view the light with their Rainbow Glasses. Instead, they will see four widely separated lines of color--one bright red, one blue-green, and two deep violet--which form hydrogen's characteristic spectrum. By contrast, a tube of neon will glow bright red. Its characteristic spectrum consists of many colorful lines, including green, yellow, and blue!
NOTE: If you do not have the necessary equipment, contact the physics or chemistry department of a local college or university. Staff are usually happy to demonstrate the use of such equipment so that you can become familiar with it. They may even be willing to lend you a power source and a few gas tubes for a brief period. Alternatively, invite a friendly faculty member to visit your school to show and tell your students about the tubes.
![]() Rainbow Glasses/Diffraction gratings:
Rainbow Glasses/Diffraction gratings:
We have suggested that students view the light source through so-called Rainbow Glasses: inexpensive cardboard spectacles with diffraction gratings in place of lenses. These can be obtained in quantity (roughly $40 per 100) from Rainbow Symphony, 6860 Canby Avenue #120, Reseda, California 91335 (phone: 1-800-821-5122).
Any kind of diffraction grating can be used successfully in this activity. Edmund Scientific Company sells inexpensive card mounted diffraction viewers (2´´ ´ 2´´) in packages of 15 (1998 Optics and Optical Instruments Catalog #D1,307: $15.50); 25 (#D39,502: $23.60); and 80 (#D50,183: $58.00). For information, contact Edmund Scientific Company, Industrial Optics Division, 101 East Gloucester Pike, Barrington, New Jersey 08007-1380 (phone: 609-573-6250).
When viewed through a diffraction grating, single-filament lamps will appear to be flanked by two series of rainbow-colored spectra, each neatly arrayed on either side of the light source. A diffraction grating has thousands of very closely spaced grooves on its surface. When light passes through these tiny grooves, it shows off its wave-like properties. Constructive and destructive interference of light waves of different lengths create repetitive, symmetrically arranged, colorful images of the light source.
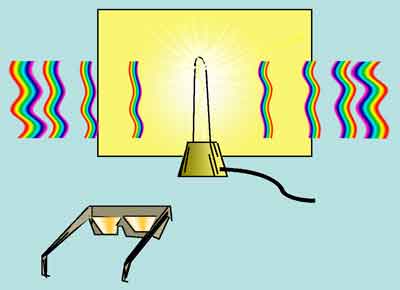
Figure 5. Appearance of rainbow-colored images seen through a diffraction grating.
Interference patterns are dependent on wavelength. Short wavelengths (violet) will interfere constructively at a distance that is closer to the light source, while long wavelengths (red) will interfere constructively farther away. This is shown schematically in Figure 6.

Figure 6. Arrangement of diffracted colors around a white-light source.
![]() Collaborative organization:
Collaborative organization:
For each pair of students, there are two clearly defined roles in the "Artists and Talkers" activity:
The two individuals will have to develop mutual understanding built on trust, clear communication, and negotiation. At first, both Artist and Talker will be encountering an unfamiliar phenomenon. To make an accurate record, the Artist must be able to imagine what the Talker sees. The Talker must be able to convert visual information into a vivid verbal picture through the use of apt analogies and clear directions. The Artist must modify the record in response to the Talker's comments and suggestions. In all of this, there will be a constant give and take .
If time permits, Artist and Talker can reverse roles and take a second set of measurements. In doing so, each should find his or her new role in the collaboration easier to handle. Each should also feel more empathetic with the challenges faced by the other. The practice the pair have had during their first collaborative effort will make this second round go much more smoothly.
e. Closure
Once again, show the "Atoms and Matter" video. Focus your students' attention on the why, what and how of the Curies' and the Hugginses' collaborations. A set of suggested discussion points is included in the lesson plan.
Encourage your students to reflect on and share their own personal experiences as collaborative partners in the "Artists and Talkers" activity. What worked? What didn't? Why? How did their experiences compare with those of other collaborative pairs in the class? How did they compare with those of the Curies and the Hugginses? What are the advantages and disadvantages of collaborative research?
f. Homework
It is common to hear people today referring to microwave cooking as "nuking" the food. This phrase has given rise to a misconception that food is being exposed to dangerous radiation similar to that associated with a nuclear weapon.
In the "Atoms and Matter" module, your students have learned how Henri Becquerel used photographic plates to detect the presence of penetrating "rays" emanating from uranium. They have simulated Becquerel's experiment using a weak radioactive source and special light-sensitive paper. They should now be able to devise a similar test to see if food cooked in a microwave has become radioactive in the process.
This assignment is intended as a unit assessment tool, to give your students an opportunity to demonstrate that they have learned the distinctive properties and behaviors of radioactive materials, as well as methods for detecting their presence. Encourage them to be creative in their experimental design.
![]()
8. APPENDIX The essay "Isotopes of Thorium" will provide you with background on the general process of radioactive decay as well as particular information on the decay of the isotope of thorium found in lantern mantles like those used in the "Radioactive 'Sandwich'" activity.
|